Transcriptional and functional effects of lithium in bipolar disorder iPSC-derived cortical spheroids
- PMID: 36653674
- PMCID: PMC10615757
- DOI: 10.1038/s41380-023-01944-0
Transcriptional and functional effects of lithium in bipolar disorder iPSC-derived cortical spheroids
Abstract
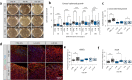
Lithium (Li) is recommended for long-term treatment of bipolar disorder (BD). However, its mechanism of action is still poorly understood. Induced pluripotent stem cell (iPSC)-derived brain organoids have emerged as a powerful tool for modeling BD-related disease mechanisms. We studied the effects of 1 mM Li treatment for 1 month in iPSC-derived human cortical spheroids (hCS) from 10 healthy controls (CTRL) and 11 BD patients (6 Li-responders, Li-R, and 5 Li non-treated, Li-N). At day 180 of differentiation, BD hCS showed smaller size, reduced proportion of neurons, decreased neuronal excitability and reduced neural network activity compared to CTRL hCS. Li rescued excitability of BD hCS neurons by exerting an opposite effect in the two diagnostic groups, increasing excitability in BD hCS and decreasing it in CTRL hCS. We identified 132 Li-associated differentially expressed genes (DEGs), which were overrepresented in sodium ion homeostasis and kidney-related pathways. Moreover, Li regulated secretion of pro-inflammatory cytokines and increased mitochondrial reserve capacity in BD hCS. Through long-term Li treatment of a human 3D brain model, this study partly elucidates the functional and transcriptional mechanisms underlying the clinical effects of Li, such as rescue of neuronal excitability and neuroprotection. Our results also underscore the substantial influence of treatment duration in Li studies. Lastly, this study illustrates the potential of patient iPSC-derived 3D brain models for precision medicine in psychiatry.
© 2023. The Author(s).
Conflict of interest statement
OAA is a consultant to HealthLytix and has received speaker’s honorarium from Lundbeck and Synovion. SD has received speaker’s honorarium from Lundbeck. The other authors declare no conflict of interest.
Figures





References
-
- Stern S, Santos R, Marchetto MC, Mendes APD, Rouleau GA, Biesmans S, et al. Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Mol Psychiatry. 2018;23:1453–65.. doi: 10.1038/mp.2016.260. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022087/Ministry of Health and Care Services | Helse Sør-Øst RHF (Southern and Eastern Norway Regional Health Authority)
- 300309/Norges Forskningsråd (Research Council of Norway)
- 223273/Norges Forskningsråd (Research Council of Norway)
- 248828/Norges Forskningsråd (Research Council of Norway)
- 80113/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)

