Live-Imaging Analysis of Epithelial Zippering During Mouse Neural Tube Closure
- PMID: 36653707
- PMCID: PMC7614165
- DOI: 10.1007/978-1-0716-2887-4_10
Live-Imaging Analysis of Epithelial Zippering During Mouse Neural Tube Closure
Abstract
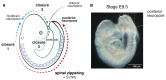
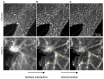
Zippering is a phenomenon of tissue morphogenesis whereby fusion between opposing epithelia progresses unidirectionally over significant distances, similar to the travel of a zip fastener, to ultimately ensure closure of an opening. A comparable process can be observed during Drosophila dorsal closure and mammalian wound healing, while zippering is employed by numerous organs such as the optic fissure, palatal shelves, tracheoesophageal foregut, and presumptive genitalia to mediate tissue sealing during normal embryonic development. Particularly striking is zippering propagation during neural tube morphogenesis, where the fusion point travels extensively along the embryonic axis to ensure closure of the neural tube. Advances in time-lapse microscopy and culture conditions have opened the opportunity for successful imaging of whole-mouse embryo development over time, providing insights into the precise cellular behavior underlying zippering propagation. Studies in mouse and the ascidian Ciona have revealed the fine-tuned cell shape changes and junction remodeling which occur at the site of zippering during neural tube morphogenesis. Here, we describe a step-by-step method for imaging at single-cell resolution the process of zippering and tissue remodeling which occurs during closure of the spinal neural tube in mouse. We also provide instructions and suggestions for quantitative morphometric analysis of cell behavior during zippering progression. This procedure can be further combined with genetic mutant models (e.g., knockouts), offering the possibility of studying the dynamics of tissue fusion and zippering propagation, which underlie a wide range of open neural tube defects.
Keywords: Cell shape; Epithelial closure; Live imaging; Neural tube; Neural tube defects; Tissue fusion.
© 2023. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

