Effect of marker-free transgenic Chlamydomonas on the control of Aedes mosquito population and on plankton
- PMID: 36653886
- PMCID: PMC9847121
- DOI: 10.1186/s13071-022-05647-3
Effect of marker-free transgenic Chlamydomonas on the control of Aedes mosquito population and on plankton
Abstract
Background: More than half of the world's population suffers from epidemic diseases that are spread by mosquitoes. The primary strategy used to stop the spread of mosquito-borne diseases is vector control. Interference RNA (RNAi) is a powerful tool for controlling insect populations and may be less susceptible to insect resistance than other strategies. However, public concerns have been raised because of the transfer of antibiotic resistance marker genes to environmental microorganisms after integration into the recipient genome, thus allowing the pathogen to acquire resistance. Therefore, in the present study, we modified the 3-hydroxykynurenine transaminase (3hkt) and hormone receptor 3 (hr3) RNAi vectors to remove antibiotic resistance marker genes and retain the expression cassette of the inverse repeat sequence of the 3hkt/hr3 target gene. This recombinant microalgal marker-free RNAi insecticide was subsequently added to the suburban water in a simulated-field trial to test its ability to control mosquito population.
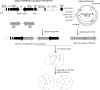
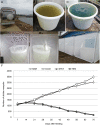
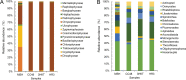
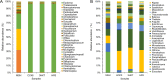
Methods: The expression cassette of the 3hkt/hr3 inverted repeat sequence and a DNA fragment of the argininosuccinate lyase gene without the ampicillin resistance gene were obtained using restriction enzyme digestion and recovery. After the cotransformation of Chlamydomonas, the recombinant algae was then employed to feed Aedes albopictus larvae. Ten and 300 larvae were used in small- and large-scale laboratory Ae.albopictus feeding trials, respectively. Simulated field trials were conducted using Meishe River water that was complemented with recombinant Chlamydomonas. Moreover, the impact of recombinant microalgae on phytoplankton and zooplankton in the released water was explored via high-throughput sequencing.
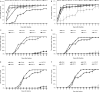
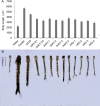
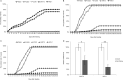
Results: The marker-free RNAi-recombinant Chlamydomonas effectively silenced the 3hkt/hr3 target gene, resulting in the inhibition of Ae. albopictus development and also in the high rate of Ae. albopictus larvae mortality in the laboratory and simulated field trials. In addition, the results confirmed that the effect of recombinant Chlamydomonas on plankton in the released water was similar to that of the nontransgenic Chlamydomonas, which could reduce the abundance and species of plankton.
Conclusions: The marker-free RNAi-recombinant Chlamydomonas are highly lethal to the Ae. albopictus mosquito, and their effect on plankton in released water is similar to that of the nontransgenic algal strains, which reduces the abundance and species of plankton. Thus, marker-free recombinant Chlamydomonas can be used for mosquito biorational control and mosquito-borne disease prevention.
Keywords: Aedes albopictus; Chlamydomonas; High-throughput sequencing; Marker-free RNA interference; Plankton.
© 2023. The Author(s).
Conflict of interest statement
The authors declare there are no conflict interests.
Figures

Similar articles
-
Development of an RNAi-based microalgal larvicide for the control of Aedes aegypti.Parasit Vectors. 2021 Aug 6;14(1):387. doi: 10.1186/s13071-021-04885-1. Parasit Vectors. 2021. PMID: 34362429 Free PMC article.
-
Controlling the development of the dengue vector Aedes aegypti using HR3 RNAi transgenic Chlamydomonas.PLoS One. 2020 Oct 14;15(10):e0240223. doi: 10.1371/journal.pone.0240223. eCollection 2020. PLoS One. 2020. PMID: 33052930 Free PMC article.
-
Control of Aedes mosquito populations using recombinant microalgae expressing short hairpin RNAs and their effect on plankton.PLoS Negl Trop Dis. 2023 Jan 26;17(1):e0011109. doi: 10.1371/journal.pntd.0011109. eCollection 2023 Jan. PLoS Negl Trop Dis. 2023. PMID: 36701378 Free PMC article.
-
RNA Interference for Mosquito and Mosquito-Borne Disease Control.Insects. 2017 Jan 5;8(1):4. doi: 10.3390/insects8010004. Insects. 2017. PMID: 28067782 Free PMC article. Review.
-
Delivery Methods for RNAi in Mosquito Larvae.J Insect Sci. 2020 Jul 1;20(4):12. doi: 10.1093/jisesa/ieaa074. J Insect Sci. 2020. PMID: 32725159 Free PMC article. Review.
Cited by
-
Engineered Gut Symbiotic Bacterium-Mediated RNAi for Effective Control of Anopheles Mosquito Larvae.Microbiol Spectr. 2023 Aug 17;11(4):e0166623. doi: 10.1128/spectrum.01666-23. Epub 2023 Jul 17. Microbiol Spectr. 2023. PMID: 37458601 Free PMC article.
-
Changes in the Stress Response and Fitness of Hybrids Between Transgenic Soybean and Wild-Type Plants Under Heat Stress.Plants (Basel). 2025 Feb 19;14(4):622. doi: 10.3390/plants14040622. Plants (Basel). 2025. PMID: 40006881 Free PMC article.
-
Use of micro and macroalgae extracts for the control of vector mosquitoes.PeerJ. 2023 Oct 9;11:e16187. doi: 10.7717/peerj.16187. eCollection 2023. PeerJ. 2023. PMID: 37842039 Free PMC article. Review.
-
Genome editing in the green alga Chlamydomonas: past, present practice and future prospects.Plant J. 2025 Apr;122(1):e70140. doi: 10.1111/tpj.70140. Plant J. 2025. PMID: 40186543 Free PMC article. Review.
References
-
- Liu QY. Dengue fever in China: new epidemical trend, challenges and strategies for prevention and control. Chin J Vector Biol Control. 2020;31:1–6.
MeSH terms
Substances
Grants and funding
- 31870344/National Natural Science Foundation of China
- 82260669/National Natural Science Foundation of China
- ZDYF2022SHFZ314/the Key Projects of Hainan Province
- NFZX2021/Financial Fund of the Ministry of Agriculture and Rural Affairs, P.R.China
- NHYYSWZZZYKZX2020/Financial Fund of the Ministry of Agriculture and Rural Affairs, P.R.China
LinkOut - more resources
Full Text Sources

