Intra-arterial Bevacizumab for Posterior Fossa Hemangioblastoma
- PMID: 36654589
- PMCID: PMC9841884
- DOI: 10.7759/cureus.32624
Intra-arterial Bevacizumab for Posterior Fossa Hemangioblastoma
Abstract
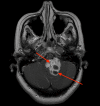
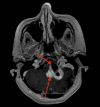
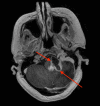
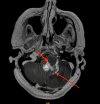
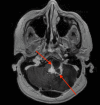
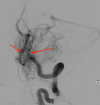
Hemangioblastoma (HB) is a rare, highly vascularized, and benign central nervous system (CNS) tumor. This vascularity is due to a high degree of signaling by vascular endothelial growth factor (VEGF). Consequently, anti-VEGF agents, such as bevacizumab, have been postulated and shown in a few cases to be effective in treating these tumors when surgical therapy is not feasible. Additionally, selective intra-arterial (IA) administration of bevacizumab has shown promise in treating other cancers such as glioblastoma (GBM). Here, we present the case of a 60-year-old female with a symptomatic posterior fossa HB where embolization and surgery were not feasible due to tumor location. She underwent selective IA treatment with bevacizumab, which led to tumor stability and symptomatic improvement. Bevacizumab has been used intravenously (IV) as a treatment for HB, however, its efficacy has not been well-established. This case demonstrates the potential viability of selective bevacizumab in HB, as demonstrated by symptomatic improvement and decreased tumor size on MRI. Further research is needed to demonstrate the specific efficacy of IA bevacizumab for CNS HB when surgery or other treatment modalities are not viable options.
Keywords: bevacizumab; endovascular; hemangioblastoma; neurosurgery; posterior fossa tumor; selective chemotherapy; von hippel landau.
Copyright © 2022, Sokol et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood-brain barrier disruption: where are we now, and where we are going. D'Amico RS, Khatri D, Reichman N, et al. J Neurooncol. 2020;147:261–278. - PubMed
-
- Antiangiogenic treatment for multiple CNS hemangioblastomas. Riklin C, Seystahl K, Hofer S, Happold C, Winterhalder R, Weller M. Onkologie. 2012;35:443–445. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
