Three-Dimensional Bioprinting of Perfusable Hierarchical Microchannels with Alginate and Silk Fibroin Double Cross-linked Network
- PMID: 36654759
- PMCID: PMC9586224
- DOI: 10.1089/3dp.2019.0115
Three-Dimensional Bioprinting of Perfusable Hierarchical Microchannels with Alginate and Silk Fibroin Double Cross-linked Network
Abstract
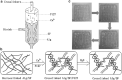
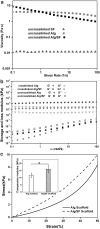
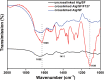
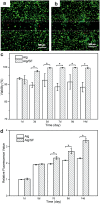
Vascularization is essential for the regeneration of three-dimensional (3D) bioprinting organs. As a general method to produce microfluidic channels in 3D printing constructs, coaxial extrusion has attracted great attention. However, the biocompatible bioinks are very limited for coaxial extrusion to fabricate microchannels with regular structure and enough mechanical properties. Herein, a hybrid bioink composed of alginate (Alg) and silk fibroin (SF) was proposed for 3D bioprinting of microchannel networks based on coaxial extrusion. The rheological properties of the bioink demonstrated that the hybrid Alg/SF bioink exhibited improved viscosity and shear thinning behavior compared with either pure Alg or SF bioink and had similar storage and loss modulus in a wide range of shear frequency, indicating a sound printability. Using a coaxial extrusion system with calcium ions and Pluronic F127 flowing through the core nozzle as cross-linkers, the Alg/SF bioink could be extruded and deposited to form a 3D scaffold with interconnected microchannels. The regular structure and smooth pore wall of microchannels inside the scaffold were demonstrated by optical coherence tomography. Micropores left by the rinse of F127 were observed by scanning electron microscope, constituting a hierarchical structure together with the microchannels and printed macropores. Fourier transform infrared spectroscopy analysis proved the complete rinse of F127 and the formation of β-sheet SF structure. Thus, Alg/SF could form a double cross-linked network, which was much stronger than the pure Alg network. Moreover, cells in the Alg/SF scaffold showed higher viability and proliferation rate than in the Alg scaffold. Therefore, Alg/SF is a promising bioink for coaxial extrusion-based 3D bioprinting, with the printed microchannel network beneficial for complex tissue and organ regeneration.
Keywords: 3D bioprinting; alginate; double cross-linked network; hierarchical microchannel; silk fibroin.
Copyright 2020, Mary Ann Liebert, Inc., publishers.
Conflict of interest statement
No competing financial interests exist.
Figures

Similar articles
-
Silk Fibroin Enhances Cytocompatibilty and Dimensional Stability of Alginate Hydrogels for Light-Based Three-Dimensional Bioprinting.Biomacromolecules. 2021 May 10;22(5):1921-1931. doi: 10.1021/acs.biomac.1c00034. Epub 2021 Apr 11. Biomacromolecules. 2021. PMID: 33840195
-
Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts.Tissue Eng Part A. 2021 Sep;27(17-18):1168-1181. doi: 10.1089/ten.TEA.2020.0305. Epub 2021 Feb 26. Tissue Eng Part A. 2021. PMID: 33218292 Free PMC article.
-
Manufacturing of self-standing multi-layered 3D-bioprinted alginate-hyaluronate constructs by controlling the cross-linking mechanisms for tissue engineering applications.Biofabrication. 2022 May 31;14(3). doi: 10.1088/1758-5090/ac6c4c. Biofabrication. 2022. PMID: 35504259
-
Silk Fibroin as a Bioink - A Thematic Review of Functionalization Strategies for Bioprinting Applications.ACS Biomater Sci Eng. 2022 Aug 8;8(8):3242-3270. doi: 10.1021/acsbiomaterials.2c00313. Epub 2022 Jul 5. ACS Biomater Sci Eng. 2022. PMID: 35786841 Review.
-
Silk Fibroin Bioinks for Digital Light Processing (DLP) 3D Bioprinting.Adv Exp Med Biol. 2020;1249:53-66. doi: 10.1007/978-981-15-3258-0_4. Adv Exp Med Biol. 2020. PMID: 32602090 Review.
Cited by
-
3D Coaxial Bioprinting: Process Mechanisms, Bioinks and Applications.Prog Biomed Eng (Bristol). 2022 Apr;4(2):022003. doi: 10.1088/2516-1091/ac631c. Epub 2022 Apr 20. Prog Biomed Eng (Bristol). 2022. PMID: 35573639 Free PMC article.
-
An Innovative Biofunctional Composite Hydrogel with Enhanced Printability, Rheological Properties, and Structural Integrity for Cell Scaffold Applications.Polymers (Basel). 2023 Jul 28;15(15):3223. doi: 10.3390/polym15153223. Polymers (Basel). 2023. PMID: 37571117 Free PMC article.
-
3D Bioprinting of Graphene Oxide-Incorporated Hydrogels for Neural Tissue Regeneration.3D Print Addit Manuf. 2024 Dec 16;11(6):e2022-e2032. doi: 10.1089/3dp.2023.0150. eCollection 2024 Dec. 3D Print Addit Manuf. 2024. PMID: 39734728
-
Formulation and Characterization of a Novel Oxidized Alginate-Gelatin-Silk Fibroin Bioink with the Aim of Skin Regeneration.Iran Biomed J. 2023 Sep 1;27(5):280-93. doi: 10.61186/ibj.27.5.280. Epub 2023 Aug 23. Iran Biomed J. 2023. PMID: 37873644 Free PMC article.
-
3D printing of tissue engineering scaffolds: a focus on vascular regeneration.Biodes Manuf. 2021;4(2):344-378. doi: 10.1007/s42242-020-00109-0. Epub 2021 Jan 4. Biodes Manuf. 2021. PMID: 33425460 Free PMC article. Review.
References
-
- Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773–785. - PubMed
-
- Lee W, Lee V, Polio S, et al. . On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol Bioeng 2010;105:1178–1186. - PubMed
-
- Luo YX, Lode A, Gelinsky M. Direct plotting of three-dimensional hollow fiber scaffolds based on concentrated alginate pastes for tissue engineering. Adv Healthc Mater 2013;2:777–783. - PubMed
-
- Gao Q, He Y, Fu JZ, et al. . Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015;61:203–215. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
