Thienopyrimidine Derivatives as GPR55 Receptor Antagonists: Insight into Structure-Activity Relationship
- PMID: 36655130
- PMCID: PMC9841585
- DOI: 10.1021/acsmedchemlett.2c00325
Thienopyrimidine Derivatives as GPR55 Receptor Antagonists: Insight into Structure-Activity Relationship
Abstract
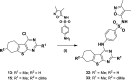
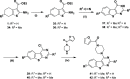
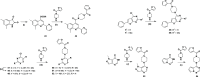
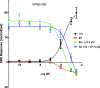
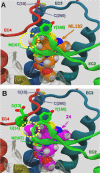
GPR55 is an orphan G-protein coupled receptor involved in various pathophysiological conditions. However, there are only a few noncannabinoid GPR55 ligands reported so far. The lack of potent and selective GPR55 ligands precludes a deep exploration of this receptor. The studies presented here focused on a thienopyrimidine scaffold based on the GPR55 antagonist ML192, previously discovered by high-throughput screening. The GPR55 activities of the new synthesized compounds were assessed using β-arrestin recruitment assays in Chinese hamster ovary cells overexpressing human GPR55. Some derivatives were identified as GPR55 antagonists with functional efficacy and selectivity versus CB1 and CB2 cannabinoid receptors.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
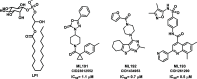


References
-
- Heynen-Genel S.; Dahl R.; Shi S.; Milan L.; Sergienko E.; Hedrick M.; Dad S.; Stonich D.; Su Y.; Chung T. D. Y.; Sharir H.; Caron M. G.; Barak L. S.; Abood M. E.. Screening for Selective Ligands for GPR55-Antagonists. Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (U.S.): Bethesda, MD, 2010. https://www.ncbi.nlm.nih.gov/books/NBK66153/ (accessed Oct 9, 2022). - PubMed
-
- Saliba S. W.; Gläser F.; Deckers A.; Keil A.; Hurrle T.; Apweiler M.; Ferver F.; Volz N.; Endres D.; Bräse S.; Fiebich B. L. Effects of a Novel Gpr55 Antagonist on the Arachidonic Acid Cascade in LPS-activated Primary Microglial Cells. Int. J. Mol. Sci. 2021, 22 (5), 2503.10.3390/ijms22052503. - DOI - PMC - PubMed
-
- Sawzdargo M.; Nguyen T.; Lee D. K.; Lynch K. R.; Cheng R.; Heng H. H.; George S. R.; O’Dowd B. F. Identification and Cloning of Three Novel Human G Protein-Coupled Receptor Genes GPR52, PsiGPR53 and GPR55: GPR55 Is Extensively Expressed in Human Brain. Mol. brain Res. 1999, 64 (2), 193–198. 10.1016/S0169-328X(98)00277-0. - DOI - PubMed
-
- Wise A.; Brown A. J.. Identification of Modulators of GPR55. WO200186305, 2001.
-
- Drmota T.; Greasley P.; Groblewski T.; Drmota P.; Greasley P.; Groblewski T.. Screening Assays for Cannabinoid- Ligand Type Modulators. WO2004074844, 2004.
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

