Magnetic Shielding Analysis of Bonding in [1.1.1]Propellane
- PMID: 36655346
- PMCID: PMC9900594
- DOI: 10.1021/acs.jpca.2c06450
Magnetic Shielding Analysis of Bonding in [1.1.1]Propellane
Abstract
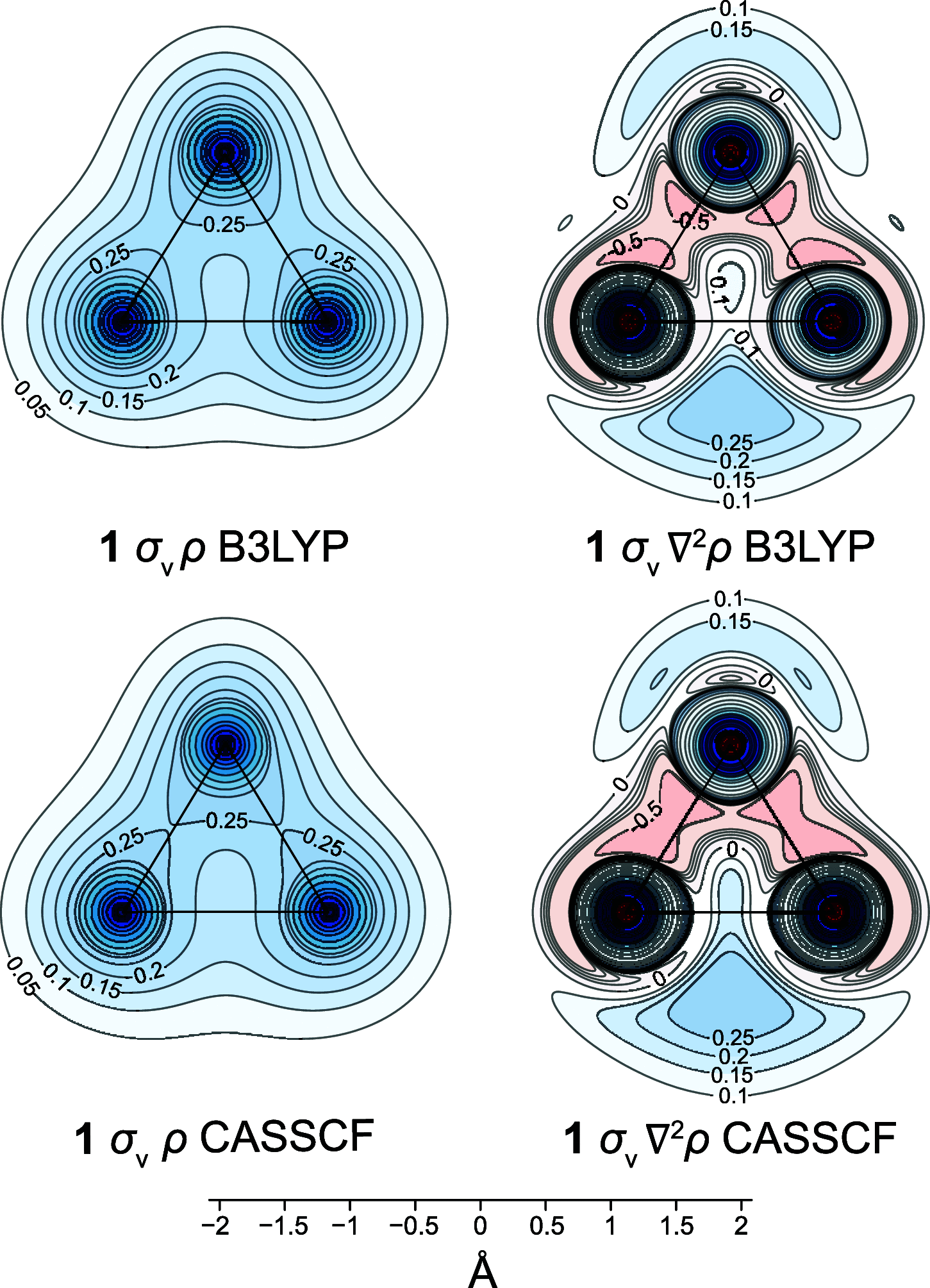
The bonding in [1.1.1]propellane, bicyclo[1.1.0]butane, bicyclo[1.1.1]pentane, tetrahedrane, and cyclopropane is investigated by analyzing changes in the off-nucleus isotropic magnetic shielding within the space surrounding each of these molecules and, for [1.1.1]propellane, by examining also the diamagnetic and paramagnetic contributions to this shielding. Any shielding arising from the two "exo" sp3-like hybrid atomic orbitals on the bridgehead carbon atoms that have been used to support the idea of an inverted bond between these two atoms is found to be almost entirely contained within the [1.1.1]propellane cage and to contribute to a strongly shielded central region. This strongly shielded region suggests the establishment of a mainly covalent bonding interaction involving all carbon atoms that cannot be straightforwardly decomposed into contributions from individual carbon-carbon bonds. The emergence of the strongly shielding central region is traced by comparing the shielding variations in and around molecules with one three-membered carbon ring (cyclopropane), two fused three-membered carbon rings (bicyclo[1.1.0]butane), and three fused three-membered carbon rings ([1.1.1]propellane).
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources

