Patient and parent-reported outcomes in paediatric ventricular assist device support: a multi-center ACTION learning network feasibility and pilot experience
- PMID: 36655506
- PMCID: PMC11285008
- DOI: 10.1017/S1047951122004048
Patient and parent-reported outcomes in paediatric ventricular assist device support: a multi-center ACTION learning network feasibility and pilot experience
Abstract
Background: Patient- and proxy-reported outcomes (PROs) are an important indicator of healthcare quality and can be used to inform treatment. Despite the widescale use of PROs in adult cardiology, they are underutilised in paediatric cardiac care. This study describes a six-center feasibility and pilot experience implementing PROs in the paediatric and young adult ventricular assist device population.
Methods: The Advanced Cardiac Therapies Improving Outcomes Network (ACTION) is a collaborative learning network comprised of 55 centres focused on improving clinical outcomes and the patient/family experience for children with heart failure and those supported by ventricular assist devices. The development of ACTION's PRO programme via engagement with patient and parent stakeholders is described. Pilot feasibility, patient/parent and clinician feedback, and initial PRO findings of patients and families receiving paediatric ventricular assist support across six centres are detailed.
Results: Thirty of the thirty-five eligible patients (85.7%) were enrolled in the PRO programme during the pilot study period. Clinicians and participating patients/parents reported positive experiences with the PRO pilot programme. The most common symptoms reported by patients/parents in the first month post-implant period included limitations in activities, dressing change distress, and post-operative pain. Poor sleep, dressing change distress, sadness, and fatigue were the most common symptoms endorsed >30 days post-implant. Parental sadness and worry were notable throughout the entirety of the post-implant experience.
Conclusions: This multi-center ACTION learning network-based PRO programme demonstrated initial success in this six-center pilot study experience and yields important next steps for larger-scale PRO collection, research, and clinical intervention.
Keywords: Patient-reported outcomes; heart failure; ventricular assist device.
Conflict of interest statement
Conflicts of Interest
None
Figures


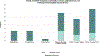
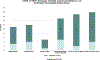

References
-
- Uzark K, Jones K, Burwinkle TM, Varni JW. The Pediatric Quality of Life Inventory™ in children with heart disease. Progress in pediatric cardiology. 2003;18(2):141–149.
-
- Pyngottu A, Werner H, Lehmann P, Balmer C. Health-related quality of life and psychological adjustment of children and adolescents with pacemakers and implantable cardioverter defibrillators: a systematic review. Pediatric cardiology. 2019;40(1):1–16. - PubMed
-
- Bevans KB, Moon J, Carle AC, et al. Patient reported outcomes as indicators of pediatric health care quality. Academic pediatrics. 2014;14(5 Suppl):S90–96. - PubMed
-
- Rumsfeld JS, Alexander KP, Goff DC Jr, et al. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127(22):2233–2249. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

