Obstetric, neonatal, and child health outcomes following embryo biopsy for preimplantation genetic testing
- PMID: 36655536
- PMCID: PMC10152168
- DOI: 10.1093/humupd/dmad001
Obstetric, neonatal, and child health outcomes following embryo biopsy for preimplantation genetic testing
Abstract
Background: Preimplantation genetic testing (PGT) of embryos developed in vitro requires a biopsy for obtaining cellular samples for the analysis. Signs of cell injury have been described in association with this procedure. Thus, the consequences of the biopsy on obstetric and neonatal outcomes have been the subject of some quantitative analyses, although the reliability of data pooling may be limited by important issues in the various reports.
Objective and rationale: The present review identifies evidence for whether pregnancies conceived after embryo biopsy are associated with a higher risk of adverse obstetric, neonatal, and long-term outcomes. Available evidence has been summarized considering manipulation at various stages of embryo development.
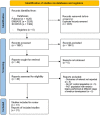
Search methods: We used the scoping review methodology. Searches of article databases were performed with keywords pertaining to the embryo biopsy technique and obstetric, neonatal, and postnatal outcomes. Studies in which embryos were biopsied at different stages (i.e. both at the cleavage and blastocyst stages) were excluded. We included data on fresh and frozen embryo transfers. The final sample of 31 documents was subjected to qualitative thematic analysis.
Outcomes: Sound evidence is lacking to fully address the issues on the potential obstetric, neonatal or long-term consequences of embryo biopsy. For polar body biopsy, the literature is too scant to draw any conclusion. Some data, although limited and controversial, suggest a possible association of embryo biopsy at the cleavage stage with an increased risk of low birthweight and small for gestational age neonates compared to babies derived from non-biopsied embryos. An increase in preterm deliveries and birth defects in cases of trophectoderm biopsy was suggested. For both biopsy methods (at the cleavage and blastocyst stages), an increased risk for hypertensive disorders of pregnancy was found. However, these findings may be explained by confounders such as other embryo manipulation procedures or by intrinsic patient or population characteristics.
Wider implications: Since there is inadequate evidence to assess obstetric, neonatal, and long-term health outcomes following embryo biopsy, an invasive PGT strategy should be developed with a cautious approach. A non-invasive approach, based on the analysis of embryo cell-free DNA, needs to be pursued to overcome the potential limitations of embryo biopsy.
Keywords: blastocyst; embryo biopsy; follow-up; maternal outcomes; neonatal outcomes; preimplantation genetic testing; scoping review; trophectoderm biopsy.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Alteri A, Corti L, Sanchez AM, Rabellotti E, Papaleo E, Viganò P.. Assessment of pre-implantation genetic testing for embryo aneuploidies: a SWOT analysis. Clin Genet 2019;95:479–487. - PubMed
-
- Archer J, Gook DA, Edgar DH.. Blastocyst formation and cell numbers in human frozen-thawed embryos following extended culture. Hum Reprod 2003;18:1669–1673. - PubMed
-
- Arksey H, O’Malley L.. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract 2005;8:19–32.
-
- Awadalla MS, Park KE, Latack KR, McGinnis LK, Ahmady A, Paulson RJ.. Influence of trophectoderm biopsy prior to frozen blastocyst transfer on obstetrical outcomes. Reprod Sci 2021;28:3459–3465. - PubMed

