Distinct role of ERp57 and ERdj5 as a disulfide isomerase and reductase during ER protein folding
- PMID: 36655611
- PMCID: PMC10022741
- DOI: 10.1242/jcs.260656
Distinct role of ERp57 and ERdj5 as a disulfide isomerase and reductase during ER protein folding
Abstract
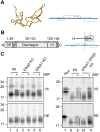
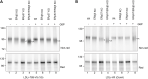
Proteins entering the secretory pathway need to attain native disulfide pairings to fold correctly. For proteins with complex disulfides, this process requires the reduction and isomerisation of non-native disulfides. Two key members of the protein disulfide isomerase (PDI) family, ERp57 and ERdj5 (also known as PDIA3 and DNAJC10, respectively), are thought to be required for correct disulfide formation but it is unknown whether they act as a reductase, an isomerase or both. In addition, it is unclear how reducing equivalents are channelled through PDI family members to substrate proteins. Here, we show that neither enzyme is required for disulfide formation, but ERp57 is required for isomerisation of non-native disulfides within glycoproteins. In addition, alternative PDIs compensate for the absence of ERp57 to isomerise glycoprotein disulfides, but only in the presence of a robust reductive pathway. ERdj5 is required for this alternative pathway to function efficiently indicating its role as a reductase. Our results define the essential cellular functions of two PDIs, highlighting a distinction between formation, reduction and isomerisation of disulfide bonds.
Keywords: Disulfide formation; Endoplasmic reticulum; Protein disulfide isomerase; Protein folding; Protein secretion.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





Similar articles
-
Thiol-disulfide exchange between the PDI family of oxidoreductases negates the requirement for an oxidase or reductase for each enzyme.Biochem J. 2015 Jul 15;469(2):279-88. doi: 10.1042/BJ20141423. Epub 2015 May 19. Biochem J. 2015. PMID: 25989104 Free PMC article.
-
Substrate specificity of the oxidoreductase ERp57 is determined primarily by its interaction with calnexin and calreticulin.J Biol Chem. 2009 Jan 23;284(4):2194-202. doi: 10.1074/jbc.M808054200. Epub 2008 Dec 3. J Biol Chem. 2009. PMID: 19054761 Free PMC article.
-
Protein disulfide isomerases (PDIs) negatively regulate ebolavirus structural glycoprotein expression in the endoplasmic reticulum (ER) via the autophagy-lysosomal pathway.Autophagy. 2022 Oct;18(10):2350-2367. doi: 10.1080/15548627.2022.2031381. Epub 2022 Feb 7. Autophagy. 2022. PMID: 35130104 Free PMC article.
-
ERp57 and PDI: multifunctional protein disulfide isomerases with similar domain architectures but differing substrate-partner associations.Biochem Cell Biol. 2006 Dec;84(6):881-9. doi: 10.1139/o06-186. Biochem Cell Biol. 2006. PMID: 17215875 Review.
-
Oxidative protein folding: from thiol-disulfide exchange reactions to the redox poise of the endoplasmic reticulum.Free Radic Biol Med. 2015 Mar;80:171-82. doi: 10.1016/j.freeradbiomed.2014.07.037. Epub 2014 Aug 1. Free Radic Biol Med. 2015. PMID: 25091901 Free PMC article. Review.
Cited by
-
Prolonged cultivation enhances the stimulatory activity of hiPSC mesenchymal progenitor-derived conditioned medium.Stem Cell Res Ther. 2024 Nov 17;15(1):434. doi: 10.1186/s13287-024-03960-5. Stem Cell Res Ther. 2024. PMID: 39551765 Free PMC article.
-
N-glycan-dependent protein maturation and quality control in the ER.Nat Rev Mol Cell Biol. 2025 May 19. doi: 10.1038/s41580-025-00855-y. Online ahead of print. Nat Rev Mol Cell Biol. 2025. PMID: 40389697 Review.
-
Protein Disulfide Isomerase Endoplasmic Reticulum Protein 57 (ERp57) is Protective Against ALS-Associated Mutant TDP-43 in Neuronal Cells.Neuromolecular Med. 2024 Jun 11;26(1):23. doi: 10.1007/s12017-024-08787-0. Neuromolecular Med. 2024. PMID: 38861223 Free PMC article.
References
-
- Addinsall, A. B., Wright, C. R., Andrikopoulos, S., Van Der Poel, C. and Stupka, N. (2018). Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease. Biochem. J. 475, 1037-1057. 10.1042/BCJ20170920 - DOI - PubMed
-
- Cao, X., Lilla, S., Cao, Z., Pringle, M. A., Oka, O. B. V., Robinson, P. J., Szmaja, T., Van Lith, M., Zanivan, S. and Bulleid, N. J. (2020). The mammalian cytosolic thioredoxin reductase pathway acts via a membrane protein to reduce ER-localised proteins. J. Cell Sci. 133, jcs241976. 10.1242/jcs.241976 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

