A Diverged Transcriptional Network for Usage of Two Fe-S Cluster Biogenesis Machineries in the Delta-Proteobacterium Myxococcus xanthus
- PMID: 36656032
- PMCID: PMC9973013
- DOI: 10.1128/mbio.03001-22
A Diverged Transcriptional Network for Usage of Two Fe-S Cluster Biogenesis Machineries in the Delta-Proteobacterium Myxococcus xanthus
Abstract
Myxococcus xanthus possesses two Fe-S cluster biogenesis machineries, ISC (iron-sulfur cluster) and SUF (sulfur mobilization). Here, we show that in comparison to the phylogenetically distant Enterobacteria, which also have both machineries, M. xanthus evolved an independent transcriptional scheme to coordinately regulate the expression of these machineries. This transcriptional response is directed by RisR, which we show to belong to a phylogenetically distant and biochemically distinct subgroup of the Rrf2 transcription factor family, in comparison to IscR that regulates the isc and suf operons in Enterobacteria. We report that RisR harbors an Fe-S cluster and that holo-RisR acts as a repressor of both the isc and suf operons, in contrast to Escherichia coli, where holo-IscR represses the isc operon whereas apo-IscR activates the suf operon. In addition, we establish that the nature of the cluster and the DNA binding sites of RisR, in the isc and suf operons, diverge from those of IscR. We further show that in M. xanthus, the two machineries appear to be fully interchangeable in maintaining housekeeping levels of Fe-S cluster biogenesis and in synthesizing the Fe-S cluster for their common regulator, RisR. We also demonstrate that in response to oxidative stress and iron limitation, transcriptional upregulation of the M. xanthus isc and suf operons was mediated solely by RisR and that the contribution of the SUF machinery was greater than the ISC machinery. Altogether, these findings shed light on the diversity of homeostatic mechanisms exploited by bacteria to coordinately use two Fe-S cluster biogenesis machineries. IMPORTANCE Fe-S proteins are ubiquitous and control a wide variety of key biological processes; therefore, maintaining Fe-S cluster homeostasis is an essential task for all organisms. Here, we provide the first example of how a bacterium from the Deltaproteobacteria branch coordinates expression of two Fe-S cluster biogenesis machineries. The results revealed a new model of coordination, highlighting the unique and common features that have independently emerged in phylogenetically distant bacteria to maintain Fe-S cluster homeostasis in response to environmental changes. Regulation is orchestrated by a previously uncharacterized transcriptional regulator, RisR, belonging to the Rrf2 superfamily, whose members are known to sense diverse environmental stresses frequently encountered by bacteria. Understanding how M. xanthus maintains Fe-S cluster homeostasis via RisR regulation revealed a strategy reflective of the aerobic lifestyle of this organsim. This new knowledge also paves the way to improve production of Fe-S-dependent secondary metabolites using M. xanthus as a chassis.
Keywords: Fe-S cluster biogenesis; Fe-S cluster homeostasis; Myxococcus xanthus; Rrf2-type regulator; iron starvation; oxidative stress; transcription regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
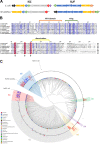
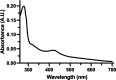

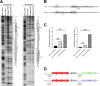
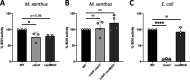


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

