High risk of infection in 'real-world' patients receiving ibrutinib, idelalisib or venetoclax for mature B-cell leukaemia/lymphoma
- PMID: 36656100
- PMCID: PMC10952205
- DOI: 10.1111/ejh.13928
High risk of infection in 'real-world' patients receiving ibrutinib, idelalisib or venetoclax for mature B-cell leukaemia/lymphoma
Abstract
Objective: The infection risk in patients receiving ibrutinib, idelalisib or venetoclax for chronic lymphocytic leukaemia (CLL) or B-cell lymphoma treated outside of clinical trials is incompletely defined. We sought to identify the severe infection rate and associated risk factors in a 'real-world' cohort.
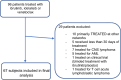
Methods: We conducted a retrospective cohort study of adult patients with CLL or lymphoma treated with ibrutinib, idelalisib or venetoclax.
Results: Of 67 patients identified (ibrutinib n = 53, idelalisib n = 8 and venetoclax n = 6), 32 (48%) experienced severe infection. Severe infection occurred at a rate of 65 infections per 100 person-years, with a median of 17.8 months of therapy. Median time to first infection (IQR) was 5.4 months (1.4-15.9). Poor baseline Eastern Cooperative Oncology Group (ECOG) performance status and high Charlson Comorbidity Index (CCI) score associated with increased risk of severe infection [hazard ratios (95% CI) 1.57 (1.07-2.31, p = .018) and 1.3 (1.05-1.62, p = .016) respectively].
Conclusion: The severe infection rate for patients receiving ibrutinib, idelalisib or venetoclax for lymphoma and CLL exceeded those reported in clinical trials. Patients with poor ECOG or high CCI should be closely monitored for early signs of infection and prevention strategies actively pursued. Further prospective research is required to define optimal antimicrobial prophylaxis recommendations.
Keywords: ibrutinib; idelalisib; infection; lymphoma; venetoclax.
© 2023 The Authors. European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
Jake Shortt has received research funding from Astex Pharmaceutical Inc., Amgen and Bristol Myers Squibb/Celgene; Jake Shortt has served on Advisory Boards for Novartis, BMS, Mundipharma and Astellas. Stephen Opat has provided consultancy to AbbVie, Astra Zeneca, Janssen and Roche; Stephen Opat has received research funding from Amgen and Beigene; Stephen Opat has received honoraria from AbbVie, Astra Zeneca, Celgene, CSL Behring, Gilead, Janssen, Merck, Roche and Takeda; Stephen Opat has served on Advisory Boards for AbbVie, Astra Zeneca, Celgene, CSL Behring, Gilead, Janssen, Merck, Roche and Takeda.
Figures
References
-
- Teh BW, Tam CS, Handunnetti S, Worth LJ, Slavin MA. Infections in patients with chronic lymphocytic leukaemia: mitigating risk in the era of targeted therapies. Blood Rev. 2018;32(6):499‐507. - PubMed
-
- Hilal T, Gea‐Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: linking mechanisms with infections. Blood Rev. 2018;32(5):387‐399. - PubMed
-
- Williams AM, Baran AM, Meacham PJ, et al. Analysis of the risk of infection in patients with chronic lymphocytic leukemia in the era of novel therapies. Leuk Lymphoma. 2018;59(3):625‐632. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources