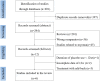
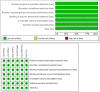
Efficacy and safety of oral gonadotropin-releasing hormone antagonists in moderate-to-severe endometriosis-associated pain: a systematic review and network meta-analysis
- PMID: 36656435
- PMCID: PMC10435625
- DOI: 10.1007/s00404-022-06862-0
Efficacy and safety of oral gonadotropin-releasing hormone antagonists in moderate-to-severe endometriosis-associated pain: a systematic review and network meta-analysis
Abstract
Purpose: The aim of this NMA is to comprehensively analyze evidence of oral GnRH antagonist in the treatment of moderate-to-severe endometriosis-associated pain.
Methods: Literature searching was performed to select eligible studies published prior to April 2022 in PubMed, Cochrane, Embase and Web of Science. Randomized controlled trials involving patients who suffered from moderate-to-severe endometriosis-associated pain and treated with oral nonpeptide GnRH antagonists or placebo were included.
Results: Elagolix 400 mg and ASP1707 15 mg were most efficient in reducing pelvic pain, dysmenorrhea and dyspareunia. Relugolix 40 mg was best in reducing the analgesics use. The rates of any TEAEs and TEAEs-related discontinuation were highest in relugolix 40 mg and elagolix 250 mg, respectively, while rates of hot flush and headache were highest in relugolix 40 mg and elagolix 150 mg. Significantly decreased spinal BMD was observed in elagolix 250 mg.
Conclusion: Oral GnRH antagonists were effective in endometriosis-associated pain in 12w, and most of the efficiency and safety outcomes were expressed in a dose-dependent manner, but linzagolix 75 mg was an exception.
Keywords: Efficiency; Endometriosis; Oral GnRH antagonists; Pain; Safety.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Oral gonadotropin-releasing hormone antagonists for treating endometriosis-associated pain: a systematic review and network meta-analysis.Fertil Steril. 2022 Dec;118(6):1102-1116. doi: 10.1016/j.fertnstert.2022.08.856. Epub 2022 Oct 22. Fertil Steril. 2022. PMID: 36283862
-
GnRH analogues and dienogest for second line treatment of endometriosis-associated pain: a systematic review, meta-analysis, and network meta-analysis.Eur J Obstet Gynecol Reprod Biol. 2025 Aug;312:114093. doi: 10.1016/j.ejogrb.2025.114093. Epub 2025 Jun 10. Eur J Obstet Gynecol Reprod Biol. 2025. PMID: 40517509
-
Progesterone receptor modulators for endometriosis.Cochrane Database Syst Rev. 2017 Jul 25;7(7):CD009881. doi: 10.1002/14651858.CD009881.pub2. Cochrane Database Syst Rev. 2017. PMID: 28742263 Free PMC article.
-
Gonadotropin-releasing hormone analogues for endometriosis.Cochrane Database Syst Rev. 2023 Jun 21;6(6):CD014788. doi: 10.1002/14651858.CD014788.pub2. Cochrane Database Syst Rev. 2023. PMID: 37341141 Free PMC article.
-
Non-inferiority study to compare the efficacy of relugolix with dienogest for endometriosis-associated pain and usefulness of administering relugolix prior to dienogest (READY study): study protocol for a multicenter randomized controlled study.Trials. 2025 Feb 6;26(1):41. doi: 10.1186/s13063-025-08750-9. Trials. 2025. PMID: 39910584 Free PMC article.
Cited by
-
Progesterone resistance in endometriosis: A pathophysiological perspective and potential treatment alternatives.Reprod Med Biol. 2024 Jun 7;23(1):e12588. doi: 10.1002/rmb2.12588. eCollection 2024 Jan-Dec. Reprod Med Biol. 2024. PMID: 38854774 Free PMC article. Review.
-
Clinical Guidance Paper VVOG: Pharmacological treatment of endometriosis-related pain.Eur J Obstet Gynecol Reprod Biol X. 2025 May 30;27:100403. doi: 10.1016/j.eurox.2025.100403. eCollection 2025 Sep. Eur J Obstet Gynecol Reprod Biol X. 2025. PMID: 40740165 Free PMC article. No abstract available.
-
Linzagolix - new perspectives in the pharmacotherapeutic management of uterine fibroids and endometriosis.Prz Menopauzalny. 2025 Jun;24(2):126-130. doi: 10.5114/pm.2025.152947. Epub 2025 Jul 28. Prz Menopauzalny. 2025. PMID: 40777872 Free PMC article. Review.
-
Efficacy of GnRH antagonists in the treatment of uterine fibroids: a meta-analysis.Arch Gynecol Obstet. 2025 Mar;311(3):685-696. doi: 10.1007/s00404-025-07932-9. Epub 2025 Jan 16. Arch Gynecol Obstet. 2025. PMID: 39821450 Free PMC article. Review.
-
Oral Gonadotropin-Releasing Hormone Antagonists in the Treatment of Endometriosis: Advances in Research.J Clin Med Res. 2025 Jun 16;17(6):299-308. doi: 10.14740/jocmr6236. eCollection 2025 Jun. J Clin Med Res. 2025. PMID: 40641861 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous