Assessment of Retinopathy of Prematurity Regression and Reactivation Using an Artificial Intelligence-Based Vascular Severity Score
- PMID: 36656578
- PMCID: PMC9857423
- DOI: 10.1001/jamanetworkopen.2022.51512
Assessment of Retinopathy of Prematurity Regression and Reactivation Using an Artificial Intelligence-Based Vascular Severity Score
Abstract
Importance: One of the biggest challenges when using anti-vascular endothelial growth factor (VEGF) agents to treat retinopathy of prematurity (ROP) is the need to perform long-term follow-up examinations to identify eyes at risk of ROP reactivation requiring retreatment.
Objective: To evaluate whether an artificial intelligence (AI)-based vascular severity score (VSS) can be used to analyze ROP regression and reactivation after anti-VEGF treatment and potentially identify eyes at risk of ROP reactivation requiring retreatment.
Design, setting, and participants: This prognostic study was a secondary analysis of posterior pole fundus images collected during the multicenter, double-blind, investigator-initiated Comparing Alternative Ranibizumab Dosages for Safety and Efficacy in Retinopathy of Prematurity (CARE-ROP) randomized clinical trial, which compared 2 different doses of ranibizumab (0.12 mg vs 0.20 mg) for the treatment of ROP. The CARE-ROP trial screened and enrolled infants between September 5, 2014, and July 14, 2016. A total of 1046 wide-angle fundus images obtained from 19 infants at predefined study time points were analyzed. The analyses of VSS were performed between January 20, 2021, and November 18, 2022.
Interventions: An AI-based algorithm assigned a VSS between 1 (normal) and 9 (most severe) to fundus images.
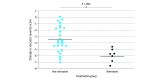
Main outcomes and measures: Analysis of VSS in infants with ROP over time and VSS comparisons between the 2 treatment groups (0.12 mg vs 0.20 mg of ranibizumab) and between infants who did and did not receive retreatment for ROP reactivation.
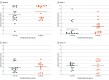
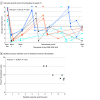
Results: Among 19 infants with ROP in the CARE-ROP randomized clinical trial, the median (range) postmenstrual age at first treatment was 36.4 (34.7-39.7) weeks; 10 infants (52.6%) were male, and 18 (94.7%) were White. The mean (SD) VSS was 6.7 (1.9) at baseline and significantly decreased to 2.7 (1.9) at week 1 (P < .001) and 2.9 (1.3) at week 4 (P < .001). The mean (SD) VSS of infants with ROP reactivation requiring retreatment was 6.5 (1.9) at the time of retreatment, which was significantly higher than the VSS at week 4 (P < .001). No significant difference was found in VSS between the 2 treatment groups, but the change in VSS between baseline and week 1 was higher for infants who later required retreatment (mean [SD], 7.8 [1.3] at baseline vs 1.7 [0.7] at week 1) vs infants who did not (mean [SD], 6.4 [1.9] at baseline vs 3.0 [2.0] at week 1). In eyes requiring retreatment, higher baseline VSS was correlated with earlier time of retreatment (Pearson r = -0.9997; P < .001).
Conclusions and relevance: In this study, VSS decreased after ranibizumab treatment, consistent with clinical disease regression. In cases of ROP reactivation requiring retreatment, VSS increased again to values comparable with baseline values. In addition, a greater change in VSS during the first week after initial treatment was found to be associated with a higher risk of later ROP reactivation, and high baseline VSS was correlated with earlier retreatment. These findings may have implications for monitoring ROP regression and reactivation after anti-VEGF treatment.
Conflict of interest statement
Figures


References
-
- Stahl A, Krohne TU, Eter N, et al. ; Comparing Alternative Ranibizumab Dosages for Safety and Efficacy in Retinopathy of Prematurity (CARE-ROP) Study Group . Comparing alternative ranibizumab dosages for safety and efficacy in retinopathy of prematurity: a randomized clinical trial. JAMA Pediatr. 2018;172(3):278-286. doi:10.1001/jamapediatrics.2017.4838 - DOI - PMC - PubMed
-
- Wallace DK, Kraker RT, Freedman SF, et al. ; Pediatric Eye Disease Investigator Group (PEDIG) . Assessment of lower doses of intravitreous bevacizumab for retinopathy of prematurity: a phase 1 dosing study. JAMA Ophthalmol. 2017;135(6):654-656. doi:10.1001/jamaophthalmol.2017.1055 - DOI - PMC - PubMed

