Receipt of Targeted Therapy and Survival Outcomes in Patients With Metastatic Colorectal Cancer
- PMID: 36656585
- PMCID: PMC9857024
- DOI: 10.1001/jamanetworkopen.2022.50030
Receipt of Targeted Therapy and Survival Outcomes in Patients With Metastatic Colorectal Cancer
Abstract
Importance: Professional society guidelines recommend treating patients with metastatic colorectal cancer with targeted therapies, including epithelial growth factor receptor (EGFR) inhibitors and vascular endothelial growth factor (VEGF) inhibitors, depending on the presence or absence of certain mutations. Since most studies of first-line targeted therapies have been limited by sample size, there is a need for larger studies using data from routine clinical care.
Objectives: To identify factors associated with receipt of first-line targeted therapies among patients with metastatic colorectal cancer for whom RAS or BRAF mutation data in the tumor were available and investigate whether targeted therapy is associated with survival.
Design, setting, and participants: This cohort study used deidentified data from an electronic health record-derived database to include patients from 800 sites of patient care across the US who were diagnosed with de novo metastatic colorectal cancer between January 1, 2013, and March 31, 2020 (n = 9134).
Main outcomes and measures: Receipt of first-line targeted therapy, categorized as ever having received EGFR inhibitors, VEGF inhibitors, or neither. The secondary outcome was overall survival.
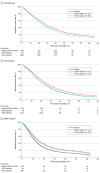
Results: The study population included 9134 patients. The median age at diagnosis was 62 years (IQR, 53-71 years), 5019 (54.9%) were male, and 5692 (62.3%) were White. The median follow-up period was 15 months. Overall, 713 patients (7.8%) received EGFR inhibitors and 5081 patients (55.6%) received VEGF inhibitors as part of their first-line treatment. Among patients with RAS wild-type (RAS-WT) tumors, 625 patients (15.5%) received EGFR inhibitors and 2053 patients (50.9%) received VEGF inhibitors. In patients with RAS mutant (RAS-Mut) tumors, 50 patients (1.1%) received EGFR inhibitors and 2682 patients (59.7%) received VEGF inhibitors; among those with BRAF-mutant (BRAF-Mut) tumors, 38 patients (6.3%) received EGFR inhibitors and 346 patients (57.2%) received VEGF inhibitors. More than one-third of the patients (36.6%) received neither EGFR inhibitors nor VEGF inhibitors. Compared with patients younger than age 40 years, those aged 80 years or older had significantly lower odds to receive targeted therapies (EGFR or VEGF inhibitors in patients with RAS-WT tumors: adjusted odds ratio [aOR], 0.53; 95% CI, 0.36-0.79; and VEGF inhibitors in patients with RAS-Mut tumors: aOR, 0.62; 95% CI, 0.42-0.90). Improved survival was associated with EGFR inhibitor therapy in patients with RAS-WT tumors (adjusted hazard ratio [aHR], 0.85; 95% CI, 0.74-0.98). Unlike in clinical trials, however, no survival benefit was noted with use of VEGF inhibitors among patients with RAS-WT (aHR, 1.00; 95% CI, 0.91-1.11) or RAS-Mut (aHR, 1.01; 95% CI, 0.93-1.10) tumors.
Conclusions and relevance: The findings of this study showed mixed results on survival benefits associated with targeted therapy. In addition, given that some of the results differed from those of randomized clinical trials, this study highlights the importance of using data originating from routine clinical care.
Conflict of interest statement
Figures
Similar articles
-
Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis.Eur J Cancer. 2015 Mar;51(5):587-94. doi: 10.1016/j.ejca.2015.01.054. Epub 2015 Feb 9. Eur J Cancer. 2015. PMID: 25673558 Review.
-
Mucinous Histology Is Associated with Resistance to Anti-EGFR Therapy in Patients with Left-Sided RAS/BRAF Wild-Type Metastatic Colorectal Cancer.Oncologist. 2022 Mar 4;27(2):104-109. doi: 10.1093/oncolo/oyab028. Oncologist. 2022. PMID: 35641204 Free PMC article.
-
Targeted Therapy for Colorectal Cancer.Surg Oncol Clin N Am. 2022 Apr;31(2):255-264. doi: 10.1016/j.soc.2021.11.006. Epub 2022 Mar 9. Surg Oncol Clin N Am. 2022. PMID: 35351276 Review.
-
Clinical Impact of PI3K/BRAF Mutations in RAS Wild Metastatic Colorectal Cancer: Meta-analysis Results.J Gastrointest Cancer. 2019 Jun;50(2):269-275. doi: 10.1007/s12029-018-0062-y. J Gastrointest Cancer. 2019. PMID: 29388061
-
Predictive and prognostic factors in the complex treatment of patients with colorectal cancer.Magy Onkol. 2010 Dec;54(4):383-94. doi: 10.1556/MOnkol.54.2010.4.13. Magy Onkol. 2010. PMID: 21163770
Cited by
-
Extraction, Purification, Structural Characteristics, Health Benefits, and Application of the Polysaccharides from Lonicera japonica Thunb.: A Review.Molecules. 2023 Jun 17;28(12):4828. doi: 10.3390/molecules28124828. Molecules. 2023. PMID: 37375383 Free PMC article. Review.
-
Cost-Effectiveness of Fruquintinib for Refractory Metastatic Colorectal Cancer in the USA.Pharmacoecon Open. 2025 Jan;9(1):93-101. doi: 10.1007/s41669-024-00529-z. Epub 2024 Oct 8. Pharmacoecon Open. 2025. PMID: 39377863 Free PMC article.
-
Biomarker Testing Approaches, Treatment Selection, and Cost of Care Among Adults With Advanced Cancer.JAMA Netw Open. 2025 Jul 1;8(7):e2519963. doi: 10.1001/jamanetworkopen.2025.19963. JAMA Netw Open. 2025. PMID: 40643914 Free PMC article.
-
Circulating miRNAs as liquid biopsy biomarkers for diagnosis in patients with colorectal cancer: a systematic review and meta-analysis.Front Genet. 2025 Jul 28;16:1574586. doi: 10.3389/fgene.2025.1574586. eCollection 2025. Front Genet. 2025. PMID: 40792072 Free PMC article.
-
Post-Operative Care of the Cancer Patient: Emphasis on Functional Recovery, Rapid Rescue, and Survivorship.Curr Oncol. 2023 Sep 19;30(9):8575-8585. doi: 10.3390/curroncol30090622. Curr Oncol. 2023. PMID: 37754537 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA043703/CA/NCI NIH HHS/United States
- R37 CA227865/CA/NCI NIH HHS/United States
- R37 CA222574/CA/NCI NIH HHS/United States
- U54 CA254566/CA/NCI NIH HHS/United States
- K12 CA076917/CA/NCI NIH HHS/United States
- P50 CA150964/CA/NCI NIH HHS/United States
- R01 CA256791/CA/NCI NIH HHS/United States
- R01 HL153175/HL/NHLBI NIH HHS/United States
- UH2 DE025487/DE/NIDCR NIH HHS/United States
- UH3 DE025487/DE/NIDCR NIH HHS/United States
- R15 NR017792/NR/NINR NIH HHS/United States
- P30 DK097948/DK/NIDDK NIH HHS/United States
- R01 AG074946/AG/NIA NIH HHS/United States
- R01 HD105892/HD/NICHD NIH HHS/United States
- U18 HS028742/HS/AHRQ HHS/United States
- U48 DP005030/DP/NCCDPHP CDC HHS/United States
- U48 DP006404/DP/NCCDPHP CDC HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

