Hypoxia enhances IPF mesenchymal progenitor cell fibrogenicity via the lactate/GPR81/HIF1α pathway
- PMID: 36656644
- PMCID: PMC9977506
- DOI: 10.1172/jci.insight.163820
Hypoxia enhances IPF mesenchymal progenitor cell fibrogenicity via the lactate/GPR81/HIF1α pathway
Abstract
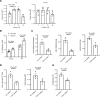
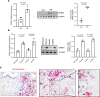
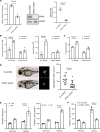
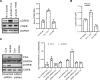
Hypoxia is a sentinel feature of idiopathic pulmonary fibrosis (IPF). The IPF microenvironment contains high lactate levels, and hypoxia enhances cellular lactate production. Lactate, acting through the GPR81 lactate receptor, serves as a signal molecule regulating cellular processes. We previously identified intrinsically fibrogenic mesenchymal progenitor cells (MPCs) that drive fibrosis in the lungs of patients with IPF. However, whether hypoxia enhances IPF MPC fibrogenicity is unclear. We hypothesized that hypoxia increases IPF MPC fibrogenicity via lactate and its cognate receptor GPR81. Here we show that hypoxia promotes IPF MPC self-renewal. The mechanism involves hypoxia-mediated enhancement of LDHA function and lactate production and release. Hypoxia also increases HIF1α levels, and this increase in turn augments the expression of GPR81. Exogenous lactate operating through GPR81 promotes IPF MPC self-renewal. IHC analysis of IPF lung tissue demonstrates IPF MPCs expressing GPR81 and hypoxic markers on the periphery of the fibroblastic focus. We show that hypoxia enhances IPF MPC fibrogenicity in vivo. We demonstrate that knockdown of GPR81 inhibits hypoxia-induced IPF MPC self-renewal in vitro and attenuates hypoxia-induced IPF MPC fibrogenicity in vivo. Our data demonstrate that hypoxia creates a feed-forward loop that augments IPF MPC fibrogenicity via the lactate/GPR81/HIF1α pathway.
Keywords: Adult stem cells; Hypoxia; Pulmonology; Stem cells.
Conflict of interest statement
Figures



References
-
- [No authors listed] American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

