Metformin acts in the gut and induces gut-liver crosstalk
- PMID: 36656866
- PMCID: PMC9942892
- DOI: 10.1073/pnas.2211933120
Metformin acts in the gut and induces gut-liver crosstalk
Abstract
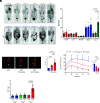
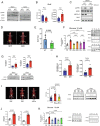
Metformin is the most prescribed drug for DM2, but its site and mechanism of action are still not well established. Here, we investigated the effects of metformin on basolateral intestinal glucose uptake (BIGU), and its consequences on hepatic glucose production (HGP). In diabetic patients and mice, the primary site of metformin action was the gut, increasing BIGU, evaluated through PET-CT. In mice and CaCo2 cells, this increase in BIGU resulted from an increase in GLUT1 and GLUT2, secondary to ATF4 and AMPK. In hyperglycemia, metformin increased the lactate (reducing pH and bicarbonate in portal vein) and acetate production in the gut, modulating liver pyruvate carboxylase, MPC1/2, and FBP1, establishing a gut-liver crosstalk that reduces HGP. In normoglycemia, metformin-induced increases in BIGU is accompanied by hypoglycemia in the portal vein, generating a counter-regulatory mechanism that avoids reductions or even increases HGP. In summary, metformin increases BIGU and through gut-liver crosstalk influences HGP.
Keywords: Diabetes; Metformin; glucose metabolism; gut-liver crosstalk.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

