Impact of list price changes on out-of-pocket costs and adherence in four high-rebate specialty drugs
- PMID: 36656871
- PMCID: PMC9851557
- DOI: 10.1371/journal.pone.0280570
Impact of list price changes on out-of-pocket costs and adherence in four high-rebate specialty drugs
Abstract
Background: Insurers manage the cost of specialty medicines via rebates, however it is unclear if the savings are passed on to patients, and whether reducing rebates may lead to changes in patient out-of-pocket (OOP) costs and medication adherence. This study examined two drug classes to understand the impact of reducing list prices to net prices, via lower-priced national drug codes (NDCs) or authorized generics, on patient OOP costs and adherence.
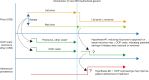
Methods: This retrospective analysis assessed IQVIA PharMetrics ® Plus adjudicated medical and pharmacy claims for commercially insured patients. Patient OOP costs per prescription and payer drug costs were assessed for evolocumab or alirocumab (proprotein convertase subtilisin/kexin type 9 inhibitors [PCSK9is]) or velpatasvir/sofosbuvir or ledipasvir/sofosbuvir (hepatitis C virus [HCV] medications). For PCSK9is and HCV medications, the original and lower-priced versions were compared. Adherence was estimated based on proportion of days covered (PDC) (PCSK9is) and receipt of full treatment regimen (HCV medications).
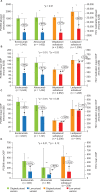
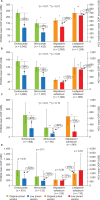
Results: In total, 10,640 patients were included (evolocumab, 5,042; alirocumab, 1,438; velpatasvir/sofosbuvir, 2,952; ledipasvir/sofosbuvir,1,208). After list price reductions, mean payer drug costs decreased by over 60%, while patient OOP cost reductions ranged from 14% to 55% (evolocumab: 55%, p < 0.01; alirocumab: 51%, p < 0.01; velpatasvir/sofosbuvir: 30%, p < 0.01; ledipasvir/sofosbuvir: 14%, p = 0.03). Patients with coinsurance as the largest contributor to their OOP costs had the largest reductions in OOP costs, ranging from adjusted, mean values of US$135 to US$379 (>60% reductions). Six-month PDC for PCSK9is and proportion receiving full HCV treatment regimen were high with the original versions and did not substantially differ with the new, lower-priced versions.
Conclusions: Reducing list prices to approximate net prices (as a proxy for reducing rebates) resulted in lower patient OOP costs, particularly for those with coinsurance. Our findings suggest that future reduction of rebates may assist in patient affordability, although additional transparency is needed.
Copyright: © 2023 Wong et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Dr Wong and Dr Seetasith are employees of Genentech, Inc. Dr Zullig has received research funding from PhRMA Foundation and Proteus Digital Health, as well as honoraria/consulting fees from Novartis and Pfizer. Dr Hung has received research funding from PhRMA Foundation and was formerly employed by Blue Cross Blue Shield Association and CVS Health. For Drs Zullig and Hung, this work was supported by the Center of Innovation for Health Services Research in Primary Care (CIN 13-410) at the Durham VA Health Care System. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Prices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis.PLoS Med. 2016 May 31;13(5):e1002032. doi: 10.1371/journal.pmed.1002032. eCollection 2016 May. PLoS Med. 2016. PMID: 27243629 Free PMC article.
-
Association of Branded Prescription Drug Rebate Size and Patient Out-of-Pocket Costs in a Nationally Representative Sample, 2007-2018.JAMA Netw Open. 2021 Jun 1;4(6):e2113393. doi: 10.1001/jamanetworkopen.2021.13393. JAMA Netw Open. 2021. PMID: 34125219 Free PMC article.
-
Estimated Changes in Manufacturer and Health Care Organization Revenue Following List Price Reductions for Hepatitis C Treatments.JAMA Netw Open. 2019 Jul 3;2(7):e196541. doi: 10.1001/jamanetworkopen.2019.6541. JAMA Netw Open. 2019. PMID: 31276176
-
Do interventions that address patient cost-sharing improve adherence to prescription drugs? A systematic review of recently published studies.Expert Rev Pharmacoecon Outcomes Res. 2019 Jun;19(3):263-277. doi: 10.1080/14737167.2019.1567335. Epub 2019 Jan 24. Expert Rev Pharmacoecon Outcomes Res. 2019. PMID: 30628493
-
Finding Truth in a World Full of Spin: Myth-Busting in the Case of Sovaldi.Clin Ther. 2015 May 1;37(5):1092-112. doi: 10.1016/j.clinthera.2015.02.009. Epub 2015 Apr 4. Clin Ther. 2015. PMID: 25850880 Review.
Cited by
-
Negotiating Medical Insurance Drug Prices: The Role in Reducing Costs of Orphan Drugs for Rare Diseases.Int J Health Policy Manag. 2023;12:8195. doi: 10.34172/ijhpm.2023.8195. Epub 2023 Nov 13. Int J Health Policy Manag. 2023. PMID: 38618767 Free PMC article. No abstract available.
References
-
- San-Juan-Rodriguez A, Piro VM, Good CB, Gellad WF, Hernandez I. Trends in list prices, net prices, and discounts of self-administered injectable tumor necrosis factor inhibitors. J Manag Care Spec Pharm. 2021;27(1):112–7. Epub 2020/12/31. doi: 10.18553/jmcp.2021.27.1.112 ; PubMed Central PMCID: PMC7788267. - DOI - PMC - PubMed
-
- San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in Prices, Market Share, and Spending on Self-administered Disease-Modifying Therapies for Multiple Sclerosis in Medicare Part D. JAMA Neurol. 2019;76(11):1386–90. Epub 2019/08/27. doi: 10.1001/jamaneurol.2019.2711 ; PubMed Central PMCID: PMC6714023. - DOI - PMC - PubMed
-
- San-Juan-Rodriguez A, Gellad WF, Good CB, Hernandez I. Trends in List Prices, Net Prices, and Discounts for Originator Biologics Facing Biosimilar Competition. JAMA Netw Open. 2019;2(12):e1917379. Epub 2019/12/14. doi: 10.1001/jamanetworkopen.2019.17379 ; PubMed Central PMCID: PMC6938676. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

