Total synthesis of lissodendoric acid A via stereospecific trapping of a strained cyclic allene
- PMID: 36656952
- PMCID: PMC10462259
- DOI: 10.1126/science.ade0032
Total synthesis of lissodendoric acid A via stereospecific trapping of a strained cyclic allene
Abstract
Small rings that contain allenes are unconventional transient compounds that have been known since the 1960s. Despite being discovered around the same time as benzyne and offering a number of synthetically advantageous features, strained cyclic allenes have seen relatively little use in chemical synthesis. We report a concise total synthesis of the manzamine alkaloid lissodendoric acid A, which hinges on the development of a regioselective, diastereoselective, and stereospecific trapping of a fleeting cyclic allene intermediate. This key step swiftly assembles the azadecalin framework of the natural product, allows for a succinct synthetic endgame, and enables a 12-step total synthesis (longest linear sequence; 0.8% overall yield). These studies demonstrate that strained cyclic allenes are versatile building blocks in chemical synthesis.
Conflict of interest statement
Figures
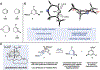


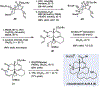
References
-
- Stoermer R, Kahlert B, Ber. Dtsch. Chem. Ges 35, 1633–1640 (1902).
-
- Roberts JD, Simmons HE Jr., L. A. Carlsmith, C. W. Vaughan, J. Am. Chem. Soc 75, 3290–3291 (1953).
-
- Wittig G, Pohmer L, Angew. Chem 67, 348 (1955).
-
- Scardiglia F, Roberts JD, Tetrahedron 1, 343–344 (1957).
-
- Wenk HH, Winkler M, Sander W, Angew. Chem. Int. Ed 42, 502–528 (2003). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

