How Many Sirtuin Genes Are Out There? Evolution of Sirtuin Genes in Vertebrates With a Description of a New Family Member
- PMID: 36656997
- PMCID: PMC9897032
- DOI: 10.1093/molbev/msad014
How Many Sirtuin Genes Are Out There? Evolution of Sirtuin Genes in Vertebrates With a Description of a New Family Member
Abstract
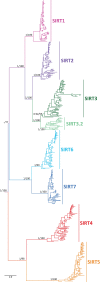
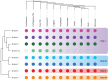
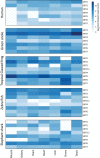
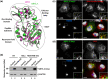
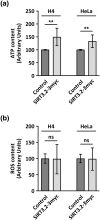
Studying the evolutionary history of gene families is a challenging and exciting task with a wide range of implications. In addition to exploring fundamental questions about the origin and evolution of genes, disentangling their evolution is also critical to those who do functional/structural studies to allow a deeper and more precise interpretation of their results in an evolutionary context. The sirtuin gene family is a group of genes that are involved in a variety of biological functions mostly related to aging. Their duplicative history is an open question, as well as the definition of the repertoire of sirtuin genes among vertebrates. Our results show a well-resolved phylogeny that represents an improvement in our understanding of the duplicative history of the sirtuin gene family. We identified a new sirtuin gene family member (SIRT3.2) that was apparently lost in the last common ancestor of amniotes but retained in all other groups of jawed vertebrates. According to our experimental analyses, elephant shark SIRT3.2 protein is located in mitochondria, the overexpression of which leads to an increase in cellular levels of ATP. Moreover, in vitro analysis demonstrated that it has deacetylase activity being modulated in a similar way to mammalian SIRT3. Our results indicate that there are at least eight sirtuin paralogs among vertebrates and that all of them can be traced back to the last common ancestor of the group that existed between 676 and 615 millions of years ago.
Keywords: SIRT; aging; deacetylase; gene duplication; gene family evolution; mitochondria.
© The Author(s) 2023. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

