Clinicopathological and predictive value of MAIT cells in non-small cell lung cancer for immunotherapy
- PMID: 36657812
- PMCID: PMC9853268
- DOI: 10.1136/jitc-2022-005902
Clinicopathological and predictive value of MAIT cells in non-small cell lung cancer for immunotherapy
Abstract
Background: Immune-checkpoint inhibitors (ICIs) remain ineffective in a large group of non-small cell lung cancer (NSCLC) patients. Mucosal-associated invariant T (MAIT) cells, a population of unconventional innate-like T lymphocytes abundant in the human body, play important roles in human malignancies. Little is known about the immune characteristics of MAIT cells in NSCLC and correlation with prognosis and response rate of ICIs treatment.
Methods: To investigate the distribution, activation status, and function of MAIT cells in NSCLC patients and their correlations with anti-PD-1 immunotherapy, MAIT cells in peripheral blood, tumor and paratumor samples from NSCLC patients with or without anti-PD-1 immunotherapy were analyzed using flow cytometry and single-cell RNA-sequencing.
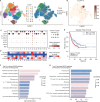
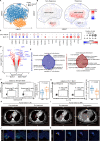
Results: MAIT cells were enriched in the tumor lesions of NSCLC patients migrating from peripheral blood via the CCR6-CCL20 axis. Both peripheral and tumor-infiltrating MAIT cells displayed an exhausted phenotype with upregulated PD-1, TIM-3, and IL-17A while less IFN-γ. Anti-PD-1 therapy reversed the function of circulating MAIT cells with higher expression of IFN-γ and granzyme B. Subcluster MAIT-17s (defined as cells highly expressing exhausted and Th17-related genes) mainly infiltrated in the non-responsive tissues, while the subcluster MAIT-IFNGRs (cells expressing genes related to cytotoxic function) were mainly enriched in responsive tissues. Moreover, we found predictive value of circulating MAIT cells for anti-PD-1 immunotherapy in NSCLC patients.
Conclusions: MAIT cells shifted to an exhausted tumor-promoting phenotype in NSCLC patients and the circulating MAIT subset could be a predictor for patients who respond to anti-PD-1 immunotherapy.
Keywords: biomarkers, tumor; immunotherapy; lung neoplasms.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures




Similar articles
-
CXCR6-positive circulating mucosal-associated invariant T cells can identify patients with non-small cell lung cancer responding to anti-PD-1 immunotherapy.J Exp Clin Cancer Res. 2024 May 3;43(1):134. doi: 10.1186/s13046-024-03046-3. J Exp Clin Cancer Res. 2024. PMID: 38698468 Free PMC article.
-
MAIT cells are associated with responsiveness to neoadjuvant immunotherapy in COPD-associated NSCLC.Cancer Med. 2024 Mar;13(6):e7112. doi: 10.1002/cam4.7112. Cancer Med. 2024. PMID: 38509769 Free PMC article.
-
Immune checkpoint blockade improves the activation and function of circulating mucosal-associated invariant T (MAIT) cells in patients with non-small cell lung cancer.Oncoimmunology. 2024 Feb 6;13(1):2312631. doi: 10.1080/2162402X.2024.2312631. eCollection 2024. Oncoimmunology. 2024. PMID: 38343750 Free PMC article.
-
Mucosal-associated invariant T cells in cancer: dual roles, complex interactions and therapeutic potential.Front Immunol. 2024 Mar 13;15:1369236. doi: 10.3389/fimmu.2024.1369236. eCollection 2024. Front Immunol. 2024. PMID: 38545100 Free PMC article. Review.
-
PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions.Oncologist. 2019 Feb;24(Suppl 1):S31-S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. Oncologist. 2019. PMID: 30819829 Free PMC article. Review.
Cited by
-
Circulating T cells: a promising biomarker of anti-PD-(L)1 therapy.Front Immunol. 2024 Mar 21;15:1371559. doi: 10.3389/fimmu.2024.1371559. eCollection 2024. Front Immunol. 2024. PMID: 38576625 Free PMC article. Review.
-
Dysfunctional states of unconventional T-cell subsets in cancer.J Leukoc Biol. 2024 Jan 5;115(1):36-46. doi: 10.1093/jleuko/qiad129. J Leukoc Biol. 2024. PMID: 37837379 Free PMC article.
-
Mucosal associated invariant T cells: Powerhouses of the lung.Immunol Lett. 2024 Oct;269:106910. doi: 10.1016/j.imlet.2024.106910. Epub 2024 Aug 10. Immunol Lett. 2024. PMID: 39128630 Free PMC article. Review.
-
Prognostic role of dynamic changes in inflammatory indicators in patients with non-small cell lung cancer treated with immune checkpoint inhibitors-a retrospective cohort study.Transl Lung Cancer Res. 2024 Aug 31;13(8):1975-1987. doi: 10.21037/tlcr-24-637. Epub 2024 Aug 28. Transl Lung Cancer Res. 2024. PMID: 39263031 Free PMC article.
-
Homologous-magnetic dual-targeted metal-organic framework to improve the Anti-hepatocellular carcinoma efficacy of PD-1 inhibitor.J Nanobiotechnology. 2024 Apr 24;22(1):206. doi: 10.1186/s12951-024-02469-6. J Nanobiotechnology. 2024. PMID: 38658950 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
