Plexin-A2 enables the proliferation and the development of tumors from glioblastoma derived cells
- PMID: 36658114
- PMCID: PMC9852426
- DOI: 10.1038/s41419-023-05554-0
Plexin-A2 enables the proliferation and the development of tumors from glioblastoma derived cells
Abstract
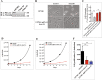
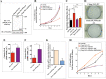
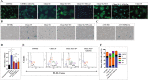
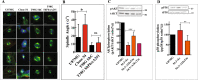
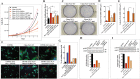
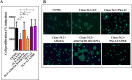
The semaphorin guidance factors receptor plexin-A2 transduces sema6A and sema6B signals and may mediate, along with plexin-A4, the anti-angiogenic effects of sema6A. When associated with neuropilins plexin-A2 also transduces the anti-angiogenic signals of sema3B. Here we show that inhibition of plexin-A2 expression in glioblastoma derived cells that express wild type p53 such as U87MG and A172 cells, or in primary human endothelial cells, strongly inhibits cell proliferation. Inhibition of plexin-A2 expression in U87MG cells also results in strong inhibition of their tumor forming ability. Knock-out of the plexin-A2 gene in U87MG cells using CRISPR/Cas9 inhibits cell proliferation which is rescued following plexin-A2 re-expression, or expression of a truncated plexin-A2 lacking its extracellular domain. Inhibition of plexin-A2 expression results in cell cycle arrest at the G2/M stage, and is accompanied by changes in cytoskeletal organization, cell flattening, and enhanced expression of senescence associated β-galactosidase. It is also associated with reduced AKT phosphorylation and enhanced phosphorylation of p38MAPK. We find that the pro-proliferative effects of plexin-A2 are mediated by FARP2 and FYN and by the GTPase activating (GAP) domain located in the intracellular domain of plexin-A2. Point mutations in these locations inhibit the rescue of cell proliferation upon re-expression of the mutated intracellular domain in the knock-out cells. In contrast re-expression of a plexin-A2 cDNA containing a point mutation in the semaphorin binding domain failed to inhibit the rescue. Our results suggest that plexin-A2 may represent a novel target for the development of anti-tumorigenic therapeutics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling.Blood. 2011 Oct 13;118(15):4285-96. doi: 10.1182/blood-2011-03-341388. Epub 2011 Aug 10. Blood. 2011. PMID: 21832283
-
The role of the plexin-A2 receptor in Sema3A and Sema3B signal transduction.J Cell Sci. 2014 Dec 15;127(Pt 24):5240-52. doi: 10.1242/jcs.155960. Epub 2014 Oct 21. J Cell Sci. 2014. PMID: 25335892
-
Semaphorin-Plexin signaling influences early ventral telencephalic development and thalamocortical axon guidance.Neural Dev. 2017 Apr 24;12(1):6. doi: 10.1186/s13064-017-0083-4. Neural Dev. 2017. PMID: 28438183 Free PMC article.
-
Roles of Sema4D and Plexin-B1 in tumor progression.Mol Cancer. 2010 Sep 21;9:251. doi: 10.1186/1476-4598-9-251. Mol Cancer. 2010. PMID: 20858260 Free PMC article. Review.
-
The evolving role of semaphorins and plexins in the immune system: Plexin-A1 regulation of dendritic cell function.Immunol Res. 2008;41(3):217-22. doi: 10.1007/s12026-008-8026-0. Immunol Res. 2008. PMID: 18649144 Review.
Cited by
-
Plexins as Regulators of Cancer Cell Proliferation, Migration, and Invasivity.Cancers (Basel). 2023 Aug 10;15(16):4046. doi: 10.3390/cancers15164046. Cancers (Basel). 2023. PMID: 37627074 Free PMC article. Review.
-
CRISPR/Cas9-Mediated Gene Therapy for Glioblastoma: A Scoping Review.Biomedicines. 2024 Jan 21;12(1):238. doi: 10.3390/biomedicines12010238. Biomedicines. 2024. PMID: 38275409 Free PMC article.
-
Genomic structural equation modeling elucidates the shared genetic architecture of allergic disorders.J Transl Med. 2025 Apr 15;23(1):445. doi: 10.1186/s12967-025-06465-8. J Transl Med. 2025. PMID: 40234965 Free PMC article.
-
Nanoparticle-Based Approaches in the Diagnosis and Treatment of Brain Tumors.J Clin Med. 2024 Dec 6;13(23):7449. doi: 10.3390/jcm13237449. J Clin Med. 2024. PMID: 39685907 Free PMC article. Review.
-
Unveiling urinary extracellular vesicle mRNA signature for early diagnosis and prognosis of bladder cancer.Theranostics. 2025 Jan 1;15(4):1272-1284. doi: 10.7150/thno.107213. eCollection 2025. Theranostics. 2025. PMID: 39816677 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

