An epithelial cell-derived metabolite tunes immunoglobulin A secretion by gut-resident plasma cells
- PMID: 36658240
- PMCID: PMC10243503
- DOI: 10.1038/s41590-022-01413-w
An epithelial cell-derived metabolite tunes immunoglobulin A secretion by gut-resident plasma cells
Abstract
Immunoglobulin A (IgA) secretion by plasma cells, terminally differentiated B cells residing in the intestinal lamina propria, assures microbiome homeostasis and protects the host against enteric infections. Exposure to diet-derived and commensal-derived signals provides immune cells with organizing cues that instruct their effector function and dynamically shape intestinal immune responses at the mucosal barrier. Recent data have described metabolic and microbial inputs controlling T cell and innate lymphoid cell activation in the gut; however, whether IgA-secreting lamina propria plasma cells are tuned by local stimuli is completely unknown. Although antibody secretion is considered to be imprinted during B cell differentiation and therefore largely unaffected by environmental changes, a rapid modulation of IgA levels in response to intestinal fluctuations might be beneficial to the host. In the present study, we showed that dietary cholesterol absorption and commensal recognition by duodenal intestinal epithelial cells lead to the production of oxysterols, evolutionarily conserved lipids with immunomodulatory functions. Using conditional cholesterol 25-hydroxylase deleter mouse line we demonstrated that 7α,25-dihydroxycholesterol from epithelial cells is critical to restrain IgA secretion against commensal- and pathogen-derived antigens in the gut. Intestinal plasma cells sense oxysterols via the chemoattractant receptor GPR183 and couple their tissue positioning with IgA secretion. Our findings revealed a new mechanism linking dietary cholesterol and humoral immune responses centered around plasma cell localization for efficient mucosal protection.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests
Figures







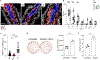
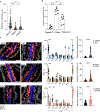





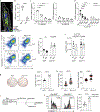
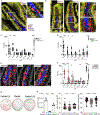

References
-
- Hand TW & Reboldi A Production and Function of Immunoglobulin A. Annual Review of Immunology 39, 695–718 (1AD). - PubMed
-
- Martinoli C, Chiavelli A & Rescigno M Entry Route of Salmonella typhimurium Directs the Type of Induced Immune Response. Immunity 27, 975–984 (2007). - PubMed
-
- Moor K et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 544, 498–502 (2017). - PubMed
-
- Fagarasan S et al. Critical Roles of Activation-Induced Cytidine Deaminase in the Homeostasis of Gut Flora. Science 298, 1424–1427 (2002). - PubMed
-
- Altmann SW et al. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science 303, 1201–1204 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

