Evolutionary diversification of methanotrophic ANME-1 archaea and their expansive virome
- PMID: 36658397
- PMCID: PMC9894754
- DOI: 10.1038/s41564-022-01297-4
Evolutionary diversification of methanotrophic ANME-1 archaea and their expansive virome
Abstract
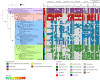
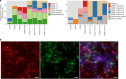
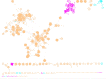
'Candidatus Methanophagales' (ANME-1) is an order-level clade of archaea responsible for anaerobic methane oxidation in deep-sea sediments. The diversity, ecology and evolution of ANME-1 remain poorly understood. In this study, we use metagenomics on deep-sea hydrothermal samples to expand ANME-1 diversity and uncover the effect of virus-host dynamics. Phylogenetic analyses reveal a deep-branching, thermophilic family, 'Candidatus Methanospirareceae', closely related to short-chain alkane oxidizers. Global phylogeny and near-complete genomes show that hydrogen metabolism within ANME-1 is an ancient trait that was vertically inherited but differentially lost during lineage diversification. Metagenomics also uncovered 16 undescribed virus families so far exclusively targeting ANME-1 archaea, showing unique structural and replicative signatures. The expansive ANME-1 virome contains a metabolic gene repertoire that can influence host ecology and evolution through virus-mediated gene displacement. Our results suggest an evolutionary continuum between anaerobic methane and short-chain alkane oxidizers and underscore the effects of viruses on the dynamics and evolution of methane-driven ecosystems.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

