Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: phase I trials
- PMID: 36658425
- PMCID: PMC11975418
- DOI: 10.1038/s41591-022-02103-8
Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: phase I trials
Abstract
Although targeting oxidative phosphorylation (OXPHOS) is a rational anticancer strategy, clinical benefit with OXPHOS inhibitors has yet to be achieved. Here we advanced IACS-010759, a highly potent and selective small-molecule complex I inhibitor, into two dose-escalation phase I trials in patients with relapsed/refractory acute myeloid leukemia (NCT02882321, n = 17) and advanced solid tumors (NCT03291938, n = 23). The primary endpoints were safety, tolerability, maximum tolerated dose and recommended phase 2 dose (RP2D) of IACS-010759. The PK, PD, and preliminary antitumor activities of IACS-010759 in patients were also evaluated as secondary endpoints in both clinical trials. IACS-010759 had a narrow therapeutic index with emergent dose-limiting toxicities, including elevated blood lactate and neurotoxicity, which obstructed efforts to maintain target exposure. Consequently no RP2D was established, only modest target inhibition and limited antitumor activity were observed at tolerated doses, and both trials were discontinued. Reverse translational studies in mice demonstrated that IACS-010759 induced behavioral and physiological changes indicative of peripheral neuropathy, which were minimized with the coadministration of a histone deacetylase 6 inhibitor. Additional studies are needed to elucidate the association between OXPHOS inhibition and neurotoxicity, and caution is warranted in the continued development of complex I inhibitors as antitumor agents.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests.
Dr. Timothy A Yap is the Medical Director of the Institute for Applied Cancer Science (M. D. Anderson Cancer Center), which has a commercial interest in DDR and other inhibitors (IACS30380/ART0380 was licensed to Artios). Dr. Yap’s research has been supported by Acrivon, Artios, AstraZeneca, Bayer, Beigene, BioNTech, Blueprint, BMS, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F-Star, GlaxoSmithKline, Genentech, Haihe, ImmuneSensor, Ionis, Ipsen, Jounce, Karyopharm, KSQ, Kyowa, Merck, Mirati, Novartis, Pfizer, Ribon Therapeutics, Regeneron, Repare, Rubius, Sanofi, Scholar Rock, Seattle Genetics, Tesaro, Vivace and Zenith; he has consulted for AbbVie, AstraZeneca, Acrivon, Adagene, Almac, Aduro, Amphista, Artios, Athena, Atrin, Avoro, Axiom, Baptist Health Systems, Bayer, Beigene, Boxer, Bristol Myers Squibb, C4 Therapeutics, Calithera, Cancer Research UK, Clovis, Cybrexa, Diffusion, EMD Serono, F-Star, Genmab, Glenmark, GLG, Globe Life Sciences, GSK, Guidepoint, Idience, Ignyta, I-Mab, ImmuneSensor, Institut Gustave Roussy, Intellisphere, Jansen, Kyn, MEI pharma, Mereo, Merck, Natera, Nexys, Novocure, OHSU, OncoSec, Ono Pharma, Pegascy, PER, Pfizer, Piper-Sandler, Prolynx, Repare, resTORbio, Roche, Schrodinger, Theragnostics, Varian, Versant, Vibliome, Xinthera, Zai Labs and ZielBio; he is a stockholder in Seagen. Dr. Naval Daver has received research funding from Daiichi-Sankyo, Bristol-Myers Squibb, Pfizer, Gilead, Sevier, Genentech, Astellas, Daiichi-Sankyo, Abbvie, Hanmi, Trovagene, FATE therapeutics, Amgen, Novimmune, Glycomimetics, Trillium, and ImmunoGen and has served in a consulting or advisory role for Daiichi-Sankyo, Bristol-Myers Squibb, Arog, Pfizer, Novartis, Jazz, Celgene, AbbVie, Astellas, Genentech, Immunogen, Servier, Syndax, Trillium, Gilead, Amgen, Shattuck labs, and Agios. Marina Konopleva has received research funding from AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven and has served in a consulting or advisory role for AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji and; Janssen. Naveen Pemmaraju serves on the Board of Directors for the following: Dan’s House of Hope; Consulting: AbbVie, Aptitude Health, Astellas Pharma US, Inc., Blueprint Medicines, Bristol-Myers Squibb, Celgene Corp, Cimeio Therapeutics AG, ClearView Healthcare Partners, CTI BioPharma, Dava Oncology, Immunogen, Incyte, Intellisphere, LLC., Novartis AG, Novartis Pharmaceuticals Corp, OncLive (Owned by Intellisphere, LLC), Patient Power, PharmaEssentia, Protagonist Therapeutics, Sanofi-aventis, Stemline Therapeutics, Inc., Total CME; Financial Relationship (e.g. Stock, Royalty, Gift, Employment or Business Ownership): Karger Publishers; Scientific/Advisory Committee Member: Cancer.Net, CareDx, CTI BioPharma, EUSA Pharma, Inc., Novartis Pharmaceuticals Corp, Pacylex, PharmaEssentia; Speaker/Preceptorship: AbbVie, Aplastic Anemia & MDS International Foundation, Curio Science LLC, Dava Oncology, Imedex, Magdalen Medical Publishing, Medscape, Neopharm, PeerView Institute for Medical Education, Physician Education Resource (PER), Physicians Education Resource (PER), Postgraduate Institute for Medicine, Stemline Therapeutics, Inc. Dr. Funda Meric-Bernstam consults for AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, Daiichi Sankyo Co. Ltd., DebioPharm, Ecor1 Capital, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., GT Apeiron, Genentech Inc., Harbinger Health, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, Menarini Group, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Protai Bio Ltd, Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks, serves on the advisory committee for Black Diamond, Biovica, Eisai, FogPharma, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Sanofi, Silverback Therapeutics, Spectrum Pharmaceuticals, and Zentalis, and has received honoraria from Chugai Biopharmaceuticals. Dr. Funda Meric-Bernstam leads clinical trials that are funded or sponsored by Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., and Taiho Pharmaceutical Co. Dr. Prithviraj Bose recieves research funding from Incyte, BMS, CTI BioPharma, Constellation (now Morphosys), Kartos, Blueprint Medicines, Cogent Biosciences, Ionis, Pfizer, Astellas, NS Pharma, and Promedior, as well as honoraria from Incyte, BMS, CTI BioPharma, Sierra Oncology (now GSK), Blueprint Medicines, Cogent Biosciences, Abbvie, Karyopharm, Pharma Essentia, Novartis, Constellation (now Morphosys) and Kartos. Dr. Apostolia M. Tsimberidou is funded by OBI Pharma, Agenus, Parker Institute for Cancer Immunotherapy, Tvardi Therapeutics, Tempus, IMMATICS; Consulting or Advisory Role: Vincerx, Diaccurate, BrYet, NEX-I, Macrogenics, and BioEclipse. Dr. Jordi R. Ahnert serves on the advisory board of Peptomyc, Kelun Pharmaceuticals/Klus Pharma, Ellipses Pharma, Molecular Partners, IONCTURA, recieves research funding from Blueprint Medicines, Black Diamond Therapeutics, Merck Sharp & Dohme, Hummingbird, Yingli, Vall d’Hebron Institute of Oncology/Cancer Core Europe, receives clinical research support from Novartis, Spectrum Pharmaceuticals, Symphogen, BioAlta, Pfizer, GenMab, CytomX, Kelun-Biotech, Takeda-Millenium, GalxoSmithKline, Taiho, Roche Pharmaceuticals, Hummingbird, Yingli, Bycicle Therapeutics, Merus, Curis, Bayer, AadiBioscience, Nuvation, ForeBio, BioMed Valley Discoveries, Loxo Oncology, Hutchinson MediPharma, Cellestia, Deciphera, Ideaya, Amgen, Tango Therapeutics, and Mirati Linnaeus Therapeutics, and receives travel support from the European Society for Medical Oncology. IACS-010759 was developed by scientists at MD Anderson. If this drug becomes FDA approved and commercially available, MD Anderson will profit from its sale. The remaining authors declare no competing interests.
Figures


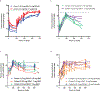
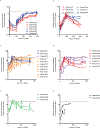




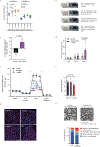


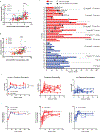

Comment in
-
Time to hit pause on mitochondria-targeting cancer therapies.Nat Med. 2023 Jan;29(1):29-30. doi: 10.1038/s41591-022-02129-y. Nat Med. 2023. PMID: 36658424 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 CA206210/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- P50 CA100632/CA/NCI NIH HHS/United States
- 1UL1TR003167/U.S. Department of Health & Human Services | NIH | Center for Information Technology (Center for Information Technology, National Institutes of Health)
- CA100632/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- CA206210/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- UL1 TR003167/TR/NCATS NIH HHS/United States
- CA227064/U.S. Department of Health & Human Services | NIH | Center for Information Technology (Center for Information Technology, National Institutes of Health)
- CA016672/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 CA227064/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

