Clinical impact of suboptimal RAASi therapy following an episode of hyperkalemia
- PMID: 36658531
- PMCID: PMC9854063
- DOI: 10.1186/s12882-022-03054-5
Clinical impact of suboptimal RAASi therapy following an episode of hyperkalemia
Abstract
Background: Hyperkalemia (HK) is a barrier to optimization of renin-angiotensin-aldosterone system inhibitor (RAASi) therapy in heart failure (HF) and chronic kidney disease (CKD). We investigated cardiorenal risk associated with changes in RAASi regimen after an episode of HK in patients with HF and/or CKD.
Methods: This observational study utilized data from hospital records, claims, and health registers from the US (Optum's de-identified Market Clarity Data) and Japan (Medical Data Vision). Included patients had an index episode of HK between July 2019 and September 2021 (US), or May 2020 and September 2021 (Japan), with prior diagnosis of HF or CKD (stage 3 or 4), and RAASi use. Risk of a cardiorenal composite outcome (HF emergency visit, HF hospitalization, or progression to end-stage kidney disease) was determined in patients who discontinued RAASi, down-titrated their dose by > 25%, or maintained or up-titrated their dose following the HK episode.
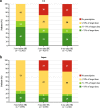
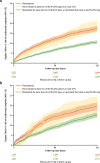
Results: A total of 15,488 and 6020 patients were included from the US and Japan, respectively. Prior to the episode of HK, 59% (US) and 27% (Japan) of patients had achieved > 50% target RAASi dose. Following the episode of HK, 33% (US) and 32% (Japan) of patients did not fill a new RAASi prescription. Risk of the cardiorenal outcome at 6 months was higher in patients who discontinued or down-titrated versus maintained or up-titrated RAASi treatment both in the US (17.5, 18.3, and 10.6%; p < 0.001) and in Japan (19.7, 20.0, and 15.1%; p < 0.001).
Conclusion: HK-related RAASi discontinuation or down-titration was associated with higher risk of cardiorenal events versus maintained or up-titrated RAASi.
Keywords: Chronic kidney disease; Guideline-directed medical therapy; Heart failure; Hyperkalemia; Potassium binder.
© 2023. The Author(s).
Conflict of interest statement
E.K. declares no competing interests.
A.R. has received institutional support relating to research grants from Alnylam Pharmaceuticals, AstraZeneca, Bayer, Gilead, GSK, Idorsia Pharmaceuticals Ltd., the National Institutes of Health, Novo Nordisk, Kadmon Corporation (LLC), Omeros Inc., Otsuka Pharmaceuticals, Palladio Biosciences, Pfizer, Protalix Biotherapeutics Ltd., Reata Pharmaceuticals, Regulus Therapeutics, Summit Therapeutics, and Sanofi; consulting fees or support for participation in advisory boards from Akebia, Amgen, Ardelyx, AstraZeneca, Aurinia, Baxter, Chiesi Global Inc., Chinook Therapeutics, Fresenius Medical Care, GSK, Otsuka, Relypsa, Sanofi, Tricida, and Vifor Pharma Inc.; speaker fees from Amgen, AstraZeneca, Aurinia, Bayer, Baxter, Fresenius Medical Care, Genzyme/Sanofi, Janssen, National Kidney Foundation, Vifor Pharma Inc., and Natera; provision of expert testimony from Quinn Emanuel Urquhart & Sullivan (LLP), and Bowman and Brooke Attorneys at Law; and, for attending meetings and/or travel from all of the above.
T.M. has received honoraria for educational materials from AstraZeneca, Lilly, and Boehringer Ingelheim Japan.
M.A. is an employee of AstraZeneca.
E.L., A.A., G.C., T.Y., and K.J. are employees of, and hold stock/stock options in, AstraZeneca.
C.V.P. has received consultancy fees from AstraZeneca.
Figures

References
-
- Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135(2):73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. - DOI - PubMed
-
- Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, Zhao N, Liu L, Lv J, Zhang H, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–741. doi: 10.1053/j.ajkd.2015.10.011. - DOI - PubMed
-
- Disease K. Improving global outcomes (KDIGO) diabetes work group: KDIGO 2020 clinical practice guideline for diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1–S115. - PubMed
-
- Disease K. Improving global outcomes blood pressure work group: KDIGO 2021 clinical practice guideline for the Management of Blood Pressure in chronic kidney Disease. Kidney Int. 2021;99(3S):S1–S87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

