Sustained Intratumoral Administration of Agonist CD40 Antibody Overcomes Immunosuppressive Tumor Microenvironment in Pancreatic Cancer
- PMID: 36658712
- PMCID: PMC10037694
- DOI: 10.1002/advs.202206873
Sustained Intratumoral Administration of Agonist CD40 Antibody Overcomes Immunosuppressive Tumor Microenvironment in Pancreatic Cancer
Abstract
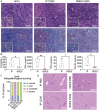
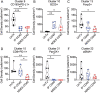
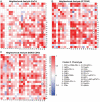
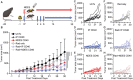
Agonist CD40 monoclonal antibodies (mAb) is a promising immunotherapeutic agent for cold-to-hot tumor immune microenvironment (TIME) conversion. Pancreatic ductal adenocarcinoma (PDAC) is an aggressive and lethal cancer known as an immune desert, and therefore urgently needs more effective treatment. Conventional systemic treatment fails to effectively penetrate the characteristic dense tumor stroma. Here, it is shown that sustained low-dose intratumoral delivery of CD40 mAb via the nanofluidic drug-eluting seed (NDES) can modulate the TIME to reduce tumor burden in murine models. NDES achieves tumor reduction at a fourfold lower dosage than systemic treatment while avoiding treatment-related adverse events. Further, abscopal responses are shown where intratumoral treatment yields growth inhibition in distant untreated tumors. Overall, the NDES is presented as a viable approach to penetrate the PDAC immune barrier in a minimally invasive and effective manner, for the overarching goal of transforming treatment.
Keywords: drug delivery; immunotherapy; implantable device; pancreatic cancer; sustained release.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Hiraoka N., Onozato K., Kosuge T., Hirohashi S., Clin. Cancer Res. 2006, 12, 5423; - PubMed
- b) Lutz E. R., Wu A. A., Bigelow E., Sharma R., Mo G., Soares K., Solt S., Dorman A., Wamwea A., Yager A., Laheru D., Wolfgang C. L., Wang J., Hruban R. H., Anders R. A., Jaffee E. M., Zheng L., Cancer Immunol. Res. 2014, 2, 616. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
