Ferroptosis-modulating small molecules for targeting drug-resistant cancer: Challenges and opportunities in manipulating redox signaling
- PMID: 36658724
- PMCID: PMC10947485
- DOI: 10.1002/med.21933
Ferroptosis-modulating small molecules for targeting drug-resistant cancer: Challenges and opportunities in manipulating redox signaling
Abstract
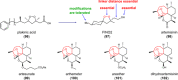
Ferroptosis is an iron-dependent cell death program that is characterized by excessive lipid peroxidation. Triggering ferroptosis has been proposed as a promising strategy to fight cancer and overcome drug resistance in antitumor therapy. Understanding the molecular interactions and structural features of ferroptosis-inducing compounds might therefore open the door to efficient pharmacological strategies against aggressive, metastatic, and therapy-resistant cancer. We here summarize the molecular mechanisms and structural requirements of ferroptosis-inducing small molecules that target central players in ferroptosis. Focus is placed on (i) glutathione peroxidase (GPX) 4, the only GPX isoenzyme that detoxifies complex membrane-bound lipid hydroperoxides, (ii) the cystine/glutamate antiporter system Xc - that is central for glutathione regeneration, (iii) the redox-protective transcription factor nuclear factor erythroid 2-related factor (NRF2), and (iv) GPX4 repression in combination with induced heme degradation via heme oxygenase-1. We deduce common features for efficient ferroptotic activity and highlight challenges in drug development. Moreover, we critically discuss the potential of natural products as ferroptosis-inducing lead structures and provide a comprehensive overview of structurally diverse biogenic and bioinspired small molecules that trigger ferroptosis via iron oxidation, inhibition of the thioredoxin/thioredoxin reductase system or less defined modes of action.
Keywords: drug-resistant cancer; ferroptosis; natural product; small molecule; structural requirement.
© 2023 The Authors. Medicinal Research Reviews published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

