Intraocular Pressure and Medication Changes Associated with Xen Gel Stent: A Systematic Review of the Literature
- PMID: 36660309
- PMCID: PMC9845068
- DOI: 10.2147/OPTH.S390955
Intraocular Pressure and Medication Changes Associated with Xen Gel Stent: A Systematic Review of the Literature
Abstract
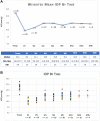
The Xen gel stent (Allergan Inc, an AbbVie company; Dublin, Ireland) was conceived as an option for patients requiring modest IOP reduction but for whom trabeculectomy was not yet indicated. As with any glaucoma surgery, establishing criteria for patient selection and identifying factors that contribute to a high likelihood of success are important. To help guide clinical decision-making, a systematic review of published studies on the gel stent was performed, with the goal of understanding postoperative outcomes based on clinical and patient factors. Results were organized around a series of pertinent clinical questions based on scenarios encountered in clinical practice. Criteria for including studies were intentionally broad, with the objective of simulating the diverse population of glaucoma patients encountered in real-world practice. Outcomes for IOP and medication reduction postoperatively were assessed in various analyses, including in eyes with various glaucoma types and severity; in eyes naïve to surgery as well as those with a history of prior incisional glaucoma surgery; and when surgery was performed as a standalone procedure or at the time of cataract surgery. The results of each of the various analyses were consistent in demonstrating that successful gel stent surgery achieved a postoperative IOP of approximately 14.0 mm Hg and reduction to fewer than 1 glaucoma medication. Additional data are shown on outcomes by method of implant (ab interno vs ab externo); intraoperative use of antifibrotics; and rates of needling in published studies.
Keywords: Xen; bleb; gel stent; glaucoma; glaucoma surgery.
© 2023 Panarelli et al.
Conflict of interest statement
Richard Fiscella is an employee of AbbVie, Inc. and may hold AbbVie stock. Brian A. Francis is a consultant for Allergan/AbbVie, Bausch & Lomb, BVI Endo Optiks, IStar, Ivantis, Iridex, MST, Thea; Speaker for Aerie. Robert J. Noecker reports consulting Fee from Alcon, Aerie, Allergan, Alimera, Beaver-Visitec, Glaukos, Iridex, Ocular Therapeutics, Santen, Thea, Kala, New World Medical, Oysterpoint, Sight Sciences, Science Branding, iStar, MST; Speakers Bureau for Allergan, Alcon, Beaver-Visitec, Iridex, Quantel, Imprimis, Kala, Nova Medical, Sight Sciences; Contracted Research for Allergan, Glaukos, Nova Medical; Equity Owner of Ocular Therapeutics, Tula Medical, ISP Surgical, Mati. Joseph F. Panarelli reports personal fees from AbbVie, Aerie, CorneaGen, Glaukos, Santen, New World Medical, Allergan, Nova Eye Medical, AOI Ophthalmics. Nathan Radcliffe reports personal fees from Alderya, AbbVie, Alcon, Allergan, Alimera, Aerie, Avellino, Bausch & Lomb, Beaver Visitec, Belkin, CATS, Carl Zeiss Meditec, Elios Vision, Equinox, Eyenovia, Eyepoint, Expert Opinion, Glaukos, Imprimis, Iridex, Ivantis, IrisVision, Lumenis, New World Medical, Novartis, Ocular Therapeutix, Regeneron, Reichert, Shire, Sight Sciences, SpyGlass, Tarsus, Thea, Tearclear, ViaLase. Arsham Sheybani is a consultant for Allergan/AbbVie, Alcon, Nova Eye, Santen, New World Medical. Oluwatosin U. Smith is a consultant for Alcon, Allergan, Iridex, Santen, New World Medical, Glaukos; Speaker & consultant for Aerie, Bausch & Lomb; Stocks from Nova Eye Medical. Vanessa Vera is an employee of AbbVie, Inc. and may hold AbbVie stock. The authors report no other conflicts of interest in this work.
Figures

References
-
- Shaarawy TM, Moschos MM, Sherwood MB. New glaucoma surgical alternatives. In: Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG, editors. Glaucoma. 2nd ed. London, England, UK: Elsevier/Saunders; 2015:1188–1201.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

