Biofilms-What Should the Orthopedic Surgeon know?
- PMID: 36660477
- PMCID: PMC9789254
- DOI: 10.1007/s43465-022-00782-6
Biofilms-What Should the Orthopedic Surgeon know?
Abstract
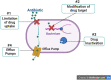
Background: Musculoskeletal infections are a major source of morbidity for orthopedic and trauma patients, are associated with prolonged treatment times, and, unfortunately, suffer from poor functional outcomes. Further complicating the issue, antimicrobial resistance (AMR) is increasingly impacting the treatment of musculoskeletal infections with a diminishing repertoire of effective antibiotic agents for some highly resistant pathogens. Most orthopedic surgical procedures involve implants, and the formation of bacterial biofilms on these implants is now recognized as a major factor contributing to the failure of antibiotic therapy in orthopedic surgery.
Methods: This review presents an overview of the types, structure, formation, and pathogenesis of biofilms as they pertain to musculoskeletal infections. Furthermore, it describes the key concepts in the management of biofilms and future perspectives for the better treatment of patients with biofilm-related musculoskeletal infections.
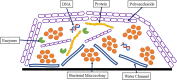
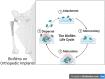
Results: A bacterial biofilm is a dynamic, living conglomerate of bacteria encased in an extracapsular polysaccharide matrix (EPS). Biofilms are a natural mode of survival for virtually all bacterial species, including both Grampositive and Gram-negative bacteria, as well as fungi. The biofilm model of growth confers resistance by several well-defined mechanisms regardless of the species of the microorganism. In most cases, biofilm management often necessitates radical measures to ensure eradication including both surgical and medical interventions.
Conclusions: Orthopedic surgeons should be aware of the key concepts pertaining to biofilms, and the impact that these can have on clinical practice.
Keywords: Antimicrobial resistance; Bacteria; Biofilm; Extracellular polymeric substance; Fracture-related infection; Musculoskeletal infection; Peri-prosthetic joint infection.
© Indian Orthopaedics Association 2022, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflicts of interestOn behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures

References
-
- Schwarz EM, Parvizi J, Gehrke T, Aiyer A, Battenberg A, Brown SA, et al. 2018 International consensus meeting on musculoskeletal infection: Research priorities from the general assembly questions. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2019;37(5):997–1006. doi: 10.1002/jor.24293. - DOI - PubMed
-
- Morgenstern M, Erichsen C, Militz M, Xie Z, Peng J, Stannard J, et al. The AO trauma CPP bone infection registry: Epidemiology and outcomes of Staphylococcus aureus bone infection. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2021;39(1):136–146. doi: 10.1002/jor.24804. - DOI - PMC - PubMed
-
- Surgical Site Infection (SSI) HAI CDC. https://www.cdc.gov/hai/ssi/ssi.html. Published Sep 28, 2021. Accessed 17.10.2022
Publication types
LinkOut - more resources
Full Text Sources
