Hericerin derivatives activates a pan-neurotrophic pathway in central hippocampal neurons converging to ERK1/2 signaling enhancing spatial memory
- PMID: 36660878
- PMCID: PMC10952766
- DOI: 10.1111/jnc.15767
Hericerin derivatives activates a pan-neurotrophic pathway in central hippocampal neurons converging to ERK1/2 signaling enhancing spatial memory
Abstract
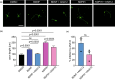
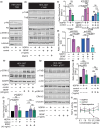
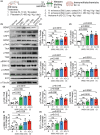
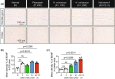
The traditional medicinal mushroom Hericium erinaceus is known for enhancing peripheral nerve regeneration through targeting nerve growth factor (NGF) neurotrophic activity. Here, we purified and identified biologically new active compounds from H. erinaceus, based on their ability to promote neurite outgrowth in hippocampal neurons. N-de phenylethyl isohericerin (NDPIH), an isoindoline compound from this mushroom, together with its hydrophobic derivative hericene A, were highly potent in promoting extensive axon outgrowth and neurite branching in cultured hippocampal neurons even in the absence of serum, demonstrating potent neurotrophic activity. Pharmacological inhibition of tropomyosin receptor kinase B (TrkB) by ANA-12 only partly prevented the NDPIH-induced neurotrophic activity, suggesting a potential link with BDNF signaling. However, we found that NDPIH activated ERK1/2 signaling in the absence of TrkB in HEK-293T cells, an effect that was not sensitive to ANA-12 in the presence of TrkB. Our results demonstrate that NDPIH acts via a complementary neurotrophic pathway independent of TrkB with converging downstream ERK1/2 activation. Mice fed with H. erinaceus crude extract and hericene A also exhibited increased neurotrophin expression and downstream signaling, resulting in significantly enhanced hippocampal memory. Hericene A therefore acts through a novel pan-neurotrophic signaling pathway, leading to improved cognitive performance.
Keywords: Hericium erinaceus; BDNF; TrkB; hericerin; memory; neurite outgrowth.
© 2023 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
This work was supported by the company CNG‐Bio inc. Frédéric A. Meunier is a former editor of the Journal of Neurochemistry.
Figures

References
-
- Bergmann, I. , Reiter, R. , Toyka, K. V. , & Koltzenburg, M. (1998). Nerve growth factor evokes hyperalgesia in mice lacking the low‐affinity neurotrophin receptor p75. Neuroscience Letters, 255, 87–90. - PubMed
-
- Brandalise, F. , Cesaroni, V. , Gregori, A. , Repetti, M. , Romano, C. , Orrù, G. , Botta, L. , Girometta, C. , Guglielminetti, M. L. , Savino, E. , & Rossi, P. (2017). Dietary supplementation of Hericium erinaceus increases mossy fiber‐CA3 hippocampal neurotransmission and recognition memory in wild‐type mice. Evidence‐Based Complementary and Alternative Medicine, 2017, 3864340–3864313. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

