Fixed-dose combination therapy-based protocol compared with free pill combination protocol: Results of a cluster randomized trial
- PMID: 36660886
- PMCID: PMC9903187
- DOI: 10.1111/jch.14632
Fixed-dose combination therapy-based protocol compared with free pill combination protocol: Results of a cluster randomized trial
Abstract
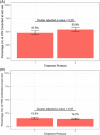
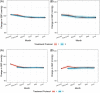
Fixed-dose combination (FDC) therapy is recommended for hypertension management in Nigeria based on randomized trials at the individual level. This cluster-randomized trial evaluates effectiveness and safety of a treatment protocol that used two-drug FDC therapy as the second and third steps for hypertension control compared with a protocol that used free pill combinations. From January 2021 to June 2021, 60 primary healthcare centers in the Federal Capital Territory of Nigeria were randomized to a protocol using FDC therapy as second and third steps compared with a protocol that used the same medications in free pill combination therapy for these steps. Eligible patients were adults (≥18 years) with hypertension. The primary outcome was the odds of a patient being controlled at their last visit between baseline to 6-month follow-up in the FDC group compared to the free pill group. 4427 patients (mean [SD] age: 49.0 [12.4] years, 70.5% female) were registered with mean (SD) baseline systolic/diastolic blood pressure 155 (20.6)/96 (13.1) mm Hg. Baseline characteristics of groups were similar. After 6-months, hypertension control rate improved in the two treatment protocols, but there were no differences between the groups after adjustment (FDC = 53.9% versus free pill combination = 47.9%, cluster-adjusted p = .29). Adverse events were similarly low (<1%) in both groups. Both protocols improved hypertension control rates at 6-months in comparison to baseline, though no differences were observed between groups. Further work is needed to determine if upfront FDC therapy is more effective and efficient to improve hypertension control rates.
Trial registration: ClinicalTrials.gov NCT04158154.
Keywords: Nigeria; cluster-randomized trial; fixed-dose combination; free pill combination; hypertension.
© 2023 The Authors. The Journal of Clinical Hypertension published by Wiley Periodicals LLC.
Conflict of interest statement
MDH has pending patents for heart failure polypills. George Health Enterprises Pty Ltd (GH) and its subsidiary, George Medicines Pty Ltd, have received investment funds to develop fixed‐dose combination products, including combinations of blood pressure‐lowering drugs. GH is the social enterprise arm of The George Institute for Global Health. Other co‐authors declare no conflicts of interest.
Figures
References
-
- Benjamin IJ, Kreutz R, Olsen MH, et al. Fixed‐dose combination antihypertensive medications. Lancet. 2019;394:637‐638. - PubMed
-
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of hypertension (ESH) and of the European society of cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

