Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial
- PMID: 36661037
- PMCID: PMC10481913
- DOI: 10.1097/SLA.0000000000005799
Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial
Abstract
Objective: To analyze risk and patterns of locoregional failure (LRF) in patients of the RAPIDO trial at 5 years.
Background: Multimodality treatment improves local control in rectal cancer. Total neoadjuvant treatment (TNT) aims to improve systemic control while local control is maintained. At 3 years, LRF rate was comparable between TNT and chemoradiotherapy in the RAPIDO trial.
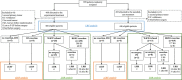
Methods: A total of 920 patients were randomized between an experimental (EXP, short-course radiotherapy, chemotherapy, and surgery) and a standard-care group (STD, chemoradiotherapy, surgery, and optional postoperative chemotherapy). LRFs, including early LRF (no resection except for organ preservation/R2 resection) and locoregional recurrence (LRR) after an R0/R1 resection, were analyzed.
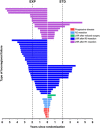
Results: Totally, 460 EXP and 446 STD patients were eligible. At 5.6 years (median follow-up), LRF was detected in 54/460 (12%) and 36/446 (8%) patients in the EXP and STD groups, respectively ( P =0.07), in which EXP patients were more often treated with 3-dimensional-conformed radiotherapy ( P =0.029). In the EXP group, LRR was detected more often [44/431 (10%) vs. 26/428 (6%); P =0.027], with more often a breached mesorectum (9/44 (21%) vs. 1/26 (4); P =0.048). The EXP treatment, enlarged lateral lymph nodes, positive circumferential resection margin, tumor deposits, and node positivity at pathology were the significant predictors for developing LRR. Location of the LRRs was similar between groups. Overall survival after LRF was comparable [hazard ratio: 0.76 (95% CI, 0.46-1.26); P =0.29].
Conclusions: The EXP treatment was associated with an increased risk of LRR, whereas the reduction in disease-related treatment failure and distant metastases remained after 5 years. Further refinement of the TNT in rectal cancer is mandated.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
P.J.N. reports honoraria from Ethicon, Johnson & Johnson, and Amgen. GAPH reports consulting fees from Roche, MSD, Amgen, and Novartis; consulting fees and research support to their institution from Bristol Myers Squibb; and research support to their institution from Seerave Foundation. A.G.H.R. and C.J.H.v.d.V. were partially funded by the EU’s Horizon 2020 research and innovation program under a Marie SkłodowskaCurie grant award (H2020MSCAITN2019, grant agreement number 857894; project acronym: CAST). M.P.H. reports consulting fees from MSD. J.C. reports consulting fees, travel expenses, and research support to their institution from Pfizer, Ipsen, and Eisai; consulting fees and research support to their institution from Bayer, Novartis, and Advanced Accelerator Applications; consulting fees from Sanofi, Exelixis, and Merck Serono; and research support to their institution from AstraZeneca. B.G. reports research support from the Swedish Cancer Society. The remaining authors report no conflicts of interest.
Figures
Comment in
-
Comment on "Locoregional Failure During and After Short-Course Radiotherapy Followed by Chemotherapy and Surgery Compared to Long-Course Chemoradiotherapy and Surgery: A Five-Year Follow-Up of the RAPIDO Trial": The RAPIDO Trial Does Not Achieve Its Primary Endpoint.Ann Surg Open. 2023 Jun 2;4(2):e288. doi: 10.1097/AS9.0000000000000288. eCollection 2023 Jun. Ann Surg Open. 2023. PMID: 37601460 Free PMC article. No abstract available.
References
-
- Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. . Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. - PubMed
-
- Bosset JF, Collette L, Calais G, et al. . Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. - PubMed
-
- Braendengen M, Tveit KM, Berglund A, et al. . Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26:3687–3694. - PubMed
-
- Bahadoer RR, Dijkstra EA, van Etten B, et al. . Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

