A critical role of RUNX1 in governing megakaryocyte-primed hematopoietic stem cell differentiation
- PMID: 36661340
- PMCID: PMC10250926
- DOI: 10.1182/bloodadvances.2022008591
A critical role of RUNX1 in governing megakaryocyte-primed hematopoietic stem cell differentiation
Abstract
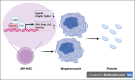
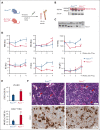
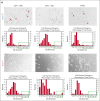
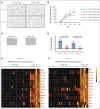
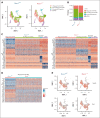
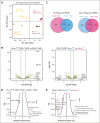
As a transcription factor in the RUNT domain core-binding factor family, RUNX1 is crucial in multiple stages of hematopoiesis, and its mutation can cause familial platelet disorder with a predisposition to acute myeloid leukemia. Previous work has established that RUNX1 is involved in the maturation of megakaryocytes (MKs) and the production of platelets. Recent studies have shown that there exists a subpopulation of hematopoietic stem cells (HSCs) with relatively high expression of von Willebrand factor and CD41 at the apex of the HSC hierarchy, termed MK-HSCs, which can give rise to MKs without going through the traditional differentiation trajectory from HSC via MPP (multipotent progenitors) and MEP (megakaryocyte-erythroid progenitor). Here, by using Runx1F/FMx1-Cre mouse model, we discovered that the MK-HSC to MK direct differentiation can occur within 1 cell division, and RUNX1 is an important regulator in the process. Runx1 knockout results in a drastic decrease in platelet counts and a severe defect in the differentiation from MK-HSCs to MKs. Single cell RNA sequencing (RNAseq) analysis shows that MK-HSCs have a distinct gene expression signature compared with non-MK-HSCs, and Runx1 deletion alters the platelet and MK-related gene expression in MK-HSCs. Furthermore, bulk RNAseq and Cut&Run analyses show that RUNX1 binds to multiple essential MK or platelet developmental genes, such as Spi1, Selp, and Itga2b and regulates their expressions in MK-HSCs. Thus, by modulating the expression of MK-related genes, RUNX1 governs the direct differentiation from MK-HSCs to MKs and platelets.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
-
- Sanjuan-Pla A, Macaulay IC, Jensen CT, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502(7470):232–236. - PubMed
-
- Benz C, Copley MR, Kent DG, et al. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell. 2012;10(3):273–283. - PubMed
-
- Song WJ, Sullivan MG, Legare RD, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23(2):166–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

