A Retrospective Study on the Efficacy of Subcutaneous Immunoglobulin as Compared to Intravenous Formulation in Patients with Chronic Lymphocytic Leukemia and Secondary Antibody Deficiency
- PMID: 36661671
- PMCID: PMC9857433
- DOI: 10.3390/curroncol30010022
A Retrospective Study on the Efficacy of Subcutaneous Immunoglobulin as Compared to Intravenous Formulation in Patients with Chronic Lymphocytic Leukemia and Secondary Antibody Deficiency
Abstract
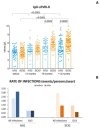
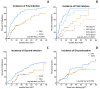
Secondary antibody deficiency (SAD) is a common complication in chronic lymphocytic leukemia (CLL) which favors the development of life-threatening infections. Subcutaneous immunoglobulins (IG) (SCIG) have been proven to be as effective as intravenous immunoglobulin (IVIG) in primary immunodeficiencies. Since only a few studies investigated SCIG in secondary antibody deficiency, the aim of this study was to assess the efficacy and safety of SCIG or IVIG in CLL patients with secondary antibody deficiency. One hundred and sixteen CLL patients were recruited, 63% were males, and the median age was 68 years; 44% had bronchiectasis and 76% never smoked. Forty-nine patients received IVIG and 88 SCIG, including 28 patients who shifted from IVIG to SCIG. Despite similar baseline IgG levels, patients receiving SCIG achieved higher IgG after at least +6 months (p = 0.0009). We observed that SCIG can decrease the cumulative incidence of first (HR 0.39 p < 0.0001) and second (HR 0.56 p = 0.0411) infection more than IVIG. The effect was remarkable in that patients were able to reach at least 6 g/L of IgG after 6 months of treatments (p < 0.0001). Replacement therapies were well tolerated with less adverse events and a lower discontinuation rate in patients was managed with SCIG than IVIG. In this study we describe the clinical features of a large cohort of CLL with secondary antibody deficiency receiving IG. We demonstrated that SCIG are active and well tolerated drugs that allows to reach higher IgG levels and decrease the rate of infections better than IVIG, in particular when IgG levels reach 6 g/L.
Keywords: chronic lymphocytic leukemia; intravenous immunoglobulin; replacement therapy; secondary immunodeficiency; subcutaneous immunoglobulin.
Conflict of interest statement
A.V. participated in the advisory boards of Janssen, Abbvie, CSL Behring, Beigene. L.T. received research funding from Gilead, Roche, Janssen and Takeda; advisory board role for Roche, Takeda, Abbvie, AstraZeneca, Octapharma; F.R.M. advisory board for Janssen, Takeda, Beigene and Abbvie; research funding from Takeda.
Figures
References
-
- Wierda W.G., Brown J., Abramson J.S., Awan F., Bilgrami S.F., Bociek G., Brander D., Chanan-Khan A.A., Coutre S.E., Davis R.S., et al. NCCN Guidelines(R) Insights: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 3.2022. J. Natl. Compr. Canc. Netw. 2022;20:622–634. doi: 10.6004/jnccn.2022.0031. - DOI - PubMed
-
- Visentin A., Mauro F.R., Cibien F., Vitale C., Reda G., Fresa A., Ciolli S., Pietrasanta D., Marchetti M., Murru R., et al. Continuous treatment with Ibrutinib in 100 untreated patients with TP53 disrupted chronic lymphocytic leukemia: A real-life campus CLL study. Am. J. Hematol. 2022;97:E95–E99. doi: 10.1002/ajh.26437. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

