Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications
- PMID: 36661802
- PMCID: PMC9858335
- DOI: 10.3390/gels9010035
Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications
Abstract
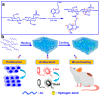
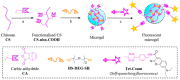
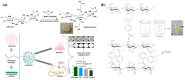
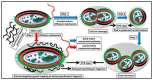
Chitosan is a prominent biopolymer in research for of its physicochemical properties and uses. Each year, the number of publications based on chitosan and its derivatives increases. Because of its comprehensive biological properties, including antibacterial, antioxidant, and tissue regeneration activities, chitosan and its derivatives can be used to prevent and treat soft tissue diseases. Furthermore, chitosan can be employed as a nanocarrier for therapeutic drug delivery. In this review, we will first discuss chitosan and chitosan-based hydrogel polymers. The structure, functionality, and physicochemical characteristics of chitosan-based hydrogels are addressed. Second, a variety of characterization approaches were used to analyze and validate the physicochemical characteristics of chitosan-based hydrogel materials. Finally, we discuss the antibacterial, antibiofilm, and antifungal uses of supramolecular chitosan-based hydrogels. This review study can be used as a base for future research into the production of various types of chitosan-based hydrogels in the antibacterial and antifungal fields.
Keywords: antibacterial activity; antibiofilm; antifungal materials; chitosan-based hydrogel; structural characterization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
-
- Rebitski E.P., Darder M. Chitosan and pectin core–shell beads encapsulating metformin–clay intercalation compounds for controlled delivery. New J. Chem. 2020;44:10102–10110. doi: 10.1039/C9NJ06433H. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

