Picomolar Detection of Lead Ions (Pb2+) by Functionally Modified Fluorescent Carbon Quantum Dots from Watermelon Juice and Their Imaging in Cancer Cells
- PMID: 36662117
- PMCID: PMC9865117
- DOI: 10.3390/jimaging9010019
Picomolar Detection of Lead Ions (Pb2+) by Functionally Modified Fluorescent Carbon Quantum Dots from Watermelon Juice and Their Imaging in Cancer Cells
Abstract
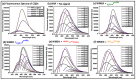
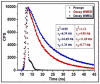
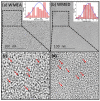
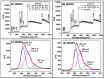
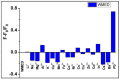
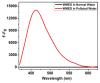
Water contamination due to the presence of lead is one of the leading causes of environmental and health hazards because of poor soil and groundwater waste management. Herein we report the synthesis of functionally modified luminescent carbon quantum dots (CQDs) obtained from watermelon juice as potential nanomaterials for the detection of toxic Pb2+ ions in polluted water and cancer cells. By introducing surface passivating ligands such as ethanolamine (EA) and ethylenediamine (ED) in watermelon juice, watermelon-ethanolamine (WMEA)-CQDs and watermelon-ethylenediamine (WMED)-CQDs exhibited a remarkable ~10-fold and ~6-fold increase in fluorescence intensity with respect to non-doped WM-CQDs. The relative fluorescence quantum yields of WMEA-CQDs and WMED-CQDs were found to be 8% and 7%, respectively, in an aqueous medium. Among various functionally-modified CQDs, only WMED-CQDs showed high selectivity towards Pb2+ ions with a remarkably good limit of detection (LoD) of 190 pM, which is less than that of the permissible limit (72 nM) in drinking water. The functionally altered WMED-CQDs detected Pb2+ metal ions in polluted water and in a human cervical cancer cell line (HeLa), thus advocating new vistas for eco-friendly nanomaterials for their use as diagnostic tools in the environment and biomedical research areas.
Keywords: bioimaging; carbon quantum dots; green synthesis; lead ion sensing; watermelon juice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- U.S. Environmental Protection Agency . Edition of the Drinking Water Standards and Health Advisories. Office of Water; Washington, DC, USA: 2009.
Grants and funding
LinkOut - more resources
Full Text Sources

