Halorotetin A: A Novel Terpenoid Compound Isolated from Ascidian Halocynthia rotetzi Exhibits the Inhibition Activity on Tumor Cell Proliferation
- PMID: 36662224
- PMCID: PMC9860651
- DOI: 10.3390/md21010051
Halorotetin A: A Novel Terpenoid Compound Isolated from Ascidian Halocynthia rotetzi Exhibits the Inhibition Activity on Tumor Cell Proliferation
Abstract
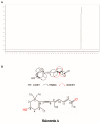
Halocynthia roretzi, the edible ascidian, has been demonstrated to be an important source of bioactive natural metabolites. Here, we reported a novel terpenoid compound named Halorotetin A that was isolated from tunic ethanol extract of H. roretzi by silica gel column chromatography, preparative layer chromatography (PLC), and semipreparative-HPLC. 1H and 13C NMRs, 1H-1H COSY, HSQC, HMBC, NOESY, and HRESIMS profiles revealed that Halorotetin A was a novel terpenoid compound with antitumor potentials. We therefore treated the culture cells with Halorotetin A and found that it significantly inhibited the proliferation of a series of tumor cells by exerting cytotoxicity, especially for the liver carcinoma cell line (HepG-2 cells). Further studies revealed that Halorotetin A affected the expression of several genes associated with the development of hepatocellular carcinoma (HCC), including oncogenes (c-myc and c-met) and HCC suppressor genes (TP53 and KEAP1). In addition, we compared the cytotoxicities of Halorotetin A and doxorubicin on HepG-2 cells. To our surprise, the cytotoxicities of Halorotetin A and doxorubicin on HepG-2 cells were similar at the same concentration and Halorotetin A did not significantly reduce the viability of the normal cells. Thus, our study identified a novel compound that significantly inhibited the proliferation of tumor cells, which provided the basis for the discovery of leading compounds for antitumor drugs.
Keywords: Halocynthia roretzi; antitumor; ascidian; hepatocellular carcinoma; terpenoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kowalski R.J., Giannakakou P., Gunasekera S.P., Longley R.E., Day B.W., Hamel E. The Microtubule-Stabilizing Agent Discodermolide Competitively Inhibits the Binding of Paclitaxel (Taxol) to Tubulin Polymers, Enhances Tubulin Nucleation Reactions More Potently than Paclitaxel, and Inhibits the Growth of Paclitaxel-Resistant Cells. Mol. Pharmacol. 1997;52:613–622. doi: 10.1124/mol.52.4.613. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

