What Role for Long-Acting Injectable Antipsychotics in Managing Schizophrenia Spectrum Disorders in Children and Adolescents? A Systematic Review
- PMID: 36662369
- PMCID: PMC9931829
- DOI: 10.1007/s40272-023-00558-x
What Role for Long-Acting Injectable Antipsychotics in Managing Schizophrenia Spectrum Disorders in Children and Adolescents? A Systematic Review
Abstract
Background: Long-acting injectable antipsychotics (LAIAs) are an efficacious and well-tolerated treatment in adults with schizophrenia spectrum disorders (SSD). However, there is less evidence for their use in children and adolescents.
Objectives: The aim of this systematic review was to summarize findings regarding the effectiveness and side effects of LAIA in children and adolescents with SSD.
Methods: Four databases (Web of Science, PubMed, MEDES, and Dialnet) were systematically searched for articles published between inception and 12 March, 2022, with the following inclusion criteria: (1) original articles or case reports; (2) providing data on efficacy/effectiveness or safety/tolerability of LAIA treatment in children and adolescents diagnosed with SSD (schizophrenia, schizoaffective disorder, schizophreniform disorder, non-affective psychotic disorder); (3) mean age of samples ≤ 18 years; and (4) written in English or Spanish. Exclusion criteria were review articles, clinical guides, expert consensus as well as posters or oral communication in conferences. The risk of bias was assessed using the ROBIS tool.
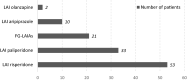
Results: From 847 articles found, 13 met the inclusion criteria. These included seven single case reports or case series, four retrospective chart reviews, a 24-week open-label trial, and one observational prospective study, covering a total of 119 adolescents (aged 12-17 years) with SSD. Almost all the articles described data on second-generation LAIA (53 patients on risperidone [once every other week], 33 on paliperidone palmitate [once monthly], 10 on aripiprazole [once monthly], and two on olanzapine pamoate [once monthly]). Twenty-one patients were reported to be only on first-generation LAIAs. Non-adherence was the main reason for starting an LAIA. In all of the studies, the use of LAIAs was associated with improvement in the patients' symptoms.
Conclusions: There are few studies assessing the use of LAIAs in adolescents with SSD. Overall, these treatments have suggested good effectiveness and acceptable safety and tolerability. However, we found no studies examining their use in children aged < 12 years. The problems and benefits linked to this type of antipsychotic formulation in the child and adolescent population require further study, ideally with prospective, controlled designs.
© 2023. The Author(s).
Conflict of interest statement
IB has received honoraria and travel support from Angelini, Otsuka-Lundbeck, and Janssen and grants from the Spanish Ministry of Health, Instituto de Salud Carlos III supported by ERDF Funds from the European Commission (PI18/0242/PI21/0391). AF has received travel and educational support from Janssen Cilag and Otsuka-Lundbeck. DI has received continuing medical education support from Rubió and Angelini. GS has received speaker fees from Angelini, and has received funding from the Alicia Koplowitz Foundation, the Brain and Behaviour Research Foundation and the Instituto de Salud Carlos III supported by ERDF Funds from the European Commission (PI1800976, PI21/00330), the Catalonia Government (2017SGR881, PERIS SLT006/17/00346), and Fundació Clínic Recerca Biomèdica (Ajut a la Recerca Pons Bartran).
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous