A single-molecule method for measuring fluorophore labeling yields for the study of membrane protein oligomerization in membranes
- PMID: 36662827
- PMCID: PMC9858377
- DOI: 10.1371/journal.pone.0280693
A single-molecule method for measuring fluorophore labeling yields for the study of membrane protein oligomerization in membranes
Abstract
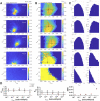
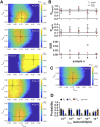
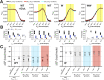
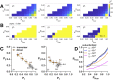
Membrane proteins are often observed as higher-order oligomers, and in some cases in multiple stoichiometric forms, raising the question of whether dynamic oligomerization can be linked to modulation of function. To better understand this potential regulatory mechanism, there is an ongoing effort to quantify equilibrium reactions of membrane protein oligomerization directly in membranes. Single-molecule photobleaching analysis is particularly useful for this as it provides a binary readout of fluorophores attached to protein subunits at dilute conditions. However, any quantification of stoichiometry also critically requires knowing the probability that a subunit is fluorescently labeled. Since labeling uncertainty is often unavoidable, we developed an approach to estimate labeling yields using the photobleaching probability distribution of an intrinsic dimeric control. By iterative fitting of an experimental dimeric photobleaching probability distribution to an expected dimer model, we estimate the fluorophore labeling yields and find agreement with direct measurements of labeling of the purified protein by UV-VIS absorbance before reconstitution. Using this labeling prediction, similar estimation methods are applied to determine the dissociation constant of reactive CLC-ec1 dimerization constructs without prior knowledge of the fluorophore labeling yield. Finally, we estimate the operational range of subunit labeling yields that allows for discrimination of monomer and dimer populations across the reactive range of mole fraction densities. Thus, our study maps out a practical method for quantifying fluorophore labeling directly from single-molecule photobleaching data, improving the ability to quantify reactive membrane protein stoichiometry in membranes.
Copyright: © 2023 Ernst et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

