Amorphous Drug-Polymer Salts: Maximizing Proton Transfer to Enhance Stability and Release
- PMID: 36668815
- PMCID: PMC9906740
- DOI: 10.1021/acs.molpharmaceut.2c00942
Amorphous Drug-Polymer Salts: Maximizing Proton Transfer to Enhance Stability and Release
Abstract
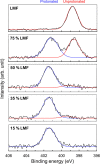
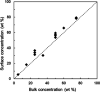
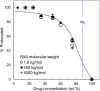
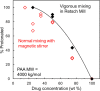
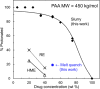
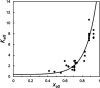
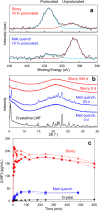
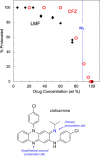
An amorphous drug-polymer salt (ADPS) can be remarkably stable against crystallization at high temperature and humidity (e.g., 40°C/75% RH) and provide fast release. Here, we report that process conditions strongly influence the degree of proton transfer (salt formation) between a drug and a polymer and in turn the product's stability and release. For lumefantrine (LMF) formulated with poly(acrylic acid) (PAA), we first show that the amorphous materials prepared by slurry conversion and antisolvent precipitation produce a single trend in which the degree of drug protonation increases with PAA concentration from 0% for pure LMF to ∼100% above 70 wt % PAA, independent of PAA's molecular weight (1.8, 450, and 4000 kg/mol). This profile describes the equilibrium for salt formation and can be modeled as a chemical equilibrium in which the basic molecules compete for the acidic groups on the polymer chain. Relative to this equilibrium, the literature methods of hot-melt extrusion (HME) and rotary evaporation (RE) reached much lower degrees of salt formation. For example, at 40 wt % drug loading, HME reached 5% salt formation and RE 15%, both well below the equilibrium value of 85%. This is noteworthy given the common use of HME and RE in manufacturing amorphous formulations, indicating a need for careful control of process conditions to ensure the full interaction between the drug and the polymer. This need arises due to the low mobility of macromolecules and the mutual hindrance of adjacent reaction sites. We find that a high degree of salt formation enhances drug stability and release. For example, at 50% drug loading, an HME-like formulation with 19% salt formation crystallized faster and released only 20% of the drug relative to a slurry-prepared formulation with 70% salt formation. Based on this work, we recommend slurry conversion as the method for preparing ADPS for its ability to enhance salt formation and continuously adjust drug loading. While this work focused on salt formation, the impact of process conditions on the molecular-level interactions between a drug and a polymer is likely a general issue for amorphous solid dispersions, with consequences on product stability and drug release.
Keywords: amorphous drug−polymer salt; dissolution; lumefantrine; physical stability; poly(acrylic acid); tropical conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Babu N. J.; Nangia A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. 10.1021/cg200492w. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

