Membrane-Disrupting Activity of Cobra Cytotoxins Is Determined by Configuration of the N-Terminal Loop
- PMID: 36668826
- PMCID: PMC9866941
- DOI: 10.3390/toxins15010006
Membrane-Disrupting Activity of Cobra Cytotoxins Is Determined by Configuration of the N-Terminal Loop
Abstract
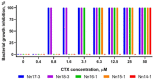
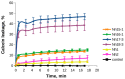
In aqueous solutions, cobra cytotoxins (CTX), three-finger folded proteins, exhibit conformational equilibrium between conformers with either cis or trans peptide bonds in the N-terminal loop (loop-I). The equilibrium is shifted to the cis form in toxins with a pair of adjacent Pro residues in this loop. It is known that CTX with a single Pro residue in loop-I and a cis peptide bond do not interact with lipid membranes. Thus, if a cis peptide bond is present in loop-I, as in a Pro-Pro containing CTX, this should weaken its lipid interactions and likely cytotoxic activities. To test this, we have isolated seven CTX from Naja naja and N. haje cobra venoms. Antibacterial and cytotoxic activities of these CTX, as well as their capability to induce calcein leakage from phospholipid liposomes, were evaluated. We have found that CTX with a Pro-Pro peptide bond indeed exhibit attenuated membrane-perturbing activity in model membranes and lower cytotoxic/antibacterial activity compared to their counterparts with a single Pro residue in loop-I.
Keywords: antibacterial activity; biological membrane; calcein leakage; cobra cytotoxin; cytotoxic activity; phospholipid liposomes; spatial structure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
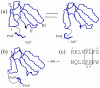




Similar articles
-
Specific Amino Acid Residues in the Three Loops of Snake Cytotoxins Determine Their Membrane Activity and Provide a Rationale for a New Classification of These Toxins.Toxins (Basel). 2024 Jun 4;16(6):262. doi: 10.3390/toxins16060262. Toxins (Basel). 2024. PMID: 38922156 Free PMC article. Review.
-
The omega-loop of cobra cytotoxins tolerates multiple amino acid substitutions.Biochem Biophys Res Commun. 2021 Jun 18;558:141-146. doi: 10.1016/j.bbrc.2021.04.069. Epub 2021 Apr 26. Biochem Biophys Res Commun. 2021. PMID: 33915327
-
Two distinct types of cardiotoxin as revealed by the structure and activity relationship of their interaction with zwitterionic phospholipid dispersions.J Biol Chem. 1994 May 20;269(20):14473-83. J Biol Chem. 1994. PMID: 8182052
-
Variability in the Spatial Structure of the Central Loop in Cobra Cytotoxins Revealed by X-ray Analysis and Molecular Modeling.Toxins (Basel). 2022 Feb 18;14(2):149. doi: 10.3390/toxins14020149. Toxins (Basel). 2022. PMID: 35202176 Free PMC article.
-
A case study of cardiotoxin III from the Taiwan cobra (Naja naja atra). Solution structure and other physical properties.Adv Exp Med Biol. 1996;391:115-29. doi: 10.1007/978-1-4613-0361-9_7. Adv Exp Med Biol. 1996. PMID: 8726052 Review. No abstract available.
Cited by
-
Fifty Years of Animal Toxin Research at the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry RAS.Int J Mol Sci. 2023 Sep 9;24(18):13884. doi: 10.3390/ijms241813884. Int J Mol Sci. 2023. PMID: 37762187 Free PMC article. Review.
-
Effects of Cobra Cardiotoxins on Intracellular Calcium and the Contracture of Rat Cardiomyocytes Depend on Their Structural Types.Int J Mol Sci. 2023 May 25;24(11):9259. doi: 10.3390/ijms24119259. Int J Mol Sci. 2023. PMID: 37298207 Free PMC article.
-
Specific Amino Acid Residues in the Three Loops of Snake Cytotoxins Determine Their Membrane Activity and Provide a Rationale for a New Classification of These Toxins.Toxins (Basel). 2024 Jun 4;16(6):262. doi: 10.3390/toxins16060262. Toxins (Basel). 2024. PMID: 38922156 Free PMC article. Review.
-
Cobra Venom Cytotoxins as a Tool for Probing Mechanisms of Mitochondrial Energetics and Understanding Mitochondrial Membrane Structure.Toxins (Basel). 2024 Jun 25;16(7):287. doi: 10.3390/toxins16070287. Toxins (Basel). 2024. PMID: 39057927 Free PMC article. Review.
-
The Role of Mitochondria in Mediation of Skeletal Muscle Repair.Muscles. 2023 Mar 24;2(2):119-163. doi: 10.3390/muscles2020011. Muscles. 2023. PMID: 40757564 Free PMC article. Review.
References
-
- Gomes A., Bhattacharjee P., Mishra R., Biswas A.K., Dasgupta S.C., Giri B. Anticancer potential of animal venoms and toxins. Ind. J. Exp. Biol. 2010;48:93–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

