The Effectiveness of Facet Joint Injection with Steroid and Botulinum Toxin in Severe Lumbar Central Spinal Stenosis: A Randomized Controlled Trial
- PMID: 36668831
- PMCID: PMC9866817
- DOI: 10.3390/toxins15010011
The Effectiveness of Facet Joint Injection with Steroid and Botulinum Toxin in Severe Lumbar Central Spinal Stenosis: A Randomized Controlled Trial
Abstract
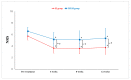
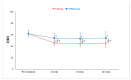
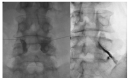
Lumbar central spinal stenosis (LCSS) is a common disorder that causes disability and pain in the elderly. It causes pain in the radicular leg. Recently, transforaminal epidural steroid injection (TFESI) has been widely used to control radicular leg pain caused by LCSS. However, in cases of severe LCSS, drugs injected using TFESI cannot spread into the spinal canal and would have less therapeutic effects than in mild LCSS. To compensate for this limitation of TFESI, we injected steroids and botulinum toxin type A into the bilateral facet joints, evaluated their effects, and compared them with those of TFESI. One hundred patients with severe LCSS were included in the study and randomly allocated to either the facet injection (FI) or TFESI group. For 50 patients in the FI group, 30 mg (40 mg/mL) of triamcinolone with 50 IU of botulinum toxin type A mixed with a 1 mL solution of 100 mL of 50% dextrose water and 30 mL of 4% lidocaine were administered into the bilateral facet joints under fluoroscopy. For 50 patients in the TFESI group, 30 mg (40 mg/mL) of triamcinolone with 0.8 mL of 2% lidocaine and 2.5 mL of 50% dextrose water was injected bilaterally under fluoroscopy. Radicular leg pain (measured with a numeric rating scale) and pain-related disability (measured with the modified Oswestry Disability Index) due to severe LCSS were significantly reduced after facet joint injection. The therapeutic effects were greater after facet joint injection than after bilateral TFESI. The injection of a mixed solution of steroids and botulinum toxin type A into the bilateral facet joints would be a beneficial therapeutic option in patients with severe LCSS.
Keywords: botulinum toxin; epidural injection; facet joint; lumbar spine; pain; radicular pain; spinal stenosis; steroid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

