Inhibition of Tolaasin Cytotoxicity Causing Brown Blotch Disease in Cultivated Mushrooms Using Tolaasin Inhibitory Factors
- PMID: 36668885
- PMCID: PMC9867037
- DOI: 10.3390/toxins15010066
Inhibition of Tolaasin Cytotoxicity Causing Brown Blotch Disease in Cultivated Mushrooms Using Tolaasin Inhibitory Factors
Abstract
Tolaasin, a pore-forming bacterial peptide toxin secreted by Pseudomonas tolaasii, causes brown blotch disease in cultivated mushrooms by forming membrane pores and collapsing the membrane structures. Tolaasin is a lipodepsipeptide, MW 1985, and pore formation by tolaasin molecules is accomplished by hydrophobic interactions and multimerizations. Compounds that inhibit tolaasin toxicity have been isolated from various food additives. Food detergents, sucrose esters of fatty acids, and polyglycerol esters of fatty acids can effectively inhibit tolaasin cytotoxicity. These chemicals, named tolaasin-inhibitory factors (TIF), were effective at concentrations ranging from 10-4 to 10-5 M. The most effective compound, TIF 16, inhibited tolaasin-induced hemolysis independent of temperature and pH, while tolaasin toxicity increased at higher temperatures. When TIF 16 was added to tolaasin-pretreated erythrocytes, the cytotoxic activity of tolaasin immediately stopped, and no further hemolysis was observed. In the artificial lipid bilayer, the single-channel activity of the tolaasin channel was completely and irreversibly blocked by TIF 16. When TIF 16 was sprayed onto pathogen-treated oyster mushrooms growing on the shelves of cultivation houses, the development of disease was completely suppressed, and normal growth of oyster mushrooms was observed. Furthermore, the treatment with TIF 16 did not show any adverse effect on the growth of oyster mushrooms. These results indicate that TIF 16 is a good candidate for the biochemical control of brown blotch disease.
Keywords: Pseudomonas tolaasii; artificial lipid bilayer; oyster mushroom (Pleurotus ostreatus); pore-forming peptide toxin; tolaasin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
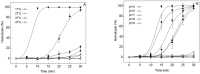
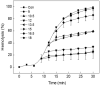
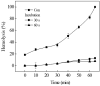

 ) and H.P. mean closed state and holding potential, respectively.
) and H.P. mean closed state and holding potential, respectively.
Similar articles
-
Temperature-dependent hemolytic activity of membrane pore-forming peptide toxin, tolaasin.J Pept Sci. 2010 Feb;16(2):85-90. doi: 10.1002/psc.1199. J Pept Sci. 2010. PMID: 19960443
-
Purification of a pore-forming peptide toxin, tolaasin, produced by Pseudomonas tolaasii 6264.J Biochem Mol Biol. 2007 Jan 31;40(1):113-8. doi: 10.5483/bmbrep.2007.40.1.113. J Biochem Mol Biol. 2007. PMID: 17244491
-
Adsorption of Tolaasins, the Toxins Behind Mushroom Bacterial Blotch, by Microbacterium spp. is Insufficient for Its Detoxification.Curr Microbiol. 2020 Jun;77(6):910-917. doi: 10.1007/s00284-020-01884-w. Epub 2020 Jan 21. Curr Microbiol. 2020. PMID: 31965226
-
100 Years Since Tolaas: Bacterial Blotch of Mushrooms in the 21st Century.Plant Dis. 2019 Nov;103(11):2714-2732. doi: 10.1094/PDIS-03-19-0589-FE. Epub 2019 Sep 27. Plant Dis. 2019. PMID: 31560599 Review.
-
Bacterial interactions with the mycelium of the cultivated edible mushrooms Agaricus bisporus and Pleurotus ostreatus.J Appl Microbiol. 2023 Jan 23;134(1):lxac018. doi: 10.1093/jambio/lxac018. J Appl Microbiol. 2023. PMID: 36626759 Review.
Cited by
-
Novel Post-Harvest Preservation Techniques for Edible Fungi: A Review.Foods. 2024 May 16;13(10):1554. doi: 10.3390/foods13101554. Foods. 2024. PMID: 38790854 Free PMC article. Review.
-
Recent Advances in the Application of Natural Products for Postharvest Edible Mushroom Quality Preservation.Foods. 2024 Jul 27;13(15):2378. doi: 10.3390/foods13152378. Foods. 2024. PMID: 39123569 Free PMC article. Review.
References
-
- Tolaas A.G. A bacterial disease of cultivated mushrooms. Phytopathology. 1915;5:51–54.
-
- Peng J.T. Ph.D. Thesis. University of Leeds; Leeds, UK: 1986. Resistance to Disease in Agaricus bisporus (lange) Imbach.
-
- Hutchison M.I., Johnstone K. Evidence for the involvement of the surface active properties of the extracellular toxin tolaasin in the manifestation of brown blotch disease symptoms by Pseudomonas tolaasii on Agaricus bisporus. Physiol. Mol. Plant. Pathol. 1993;42:273–384. doi: 10.1016/S0885-5765(05)80013-X. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

