Montane Rattlesnakes in México: Venoms of Crotalus tancitarensis and Related Species within the Crotalus intermedius Group
- PMID: 36668891
- PMCID: PMC9867100
- DOI: 10.3390/toxins15010072
Montane Rattlesnakes in México: Venoms of Crotalus tancitarensis and Related Species within the Crotalus intermedius Group
Abstract
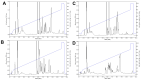
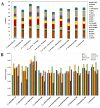
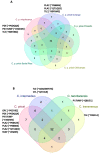
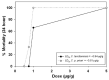
The Crotalus intermedius group is a clade of rattlesnakes consisting of several species adapted to a high elevation habitat, primarily in México. Crotalus tancitarensis was previously classified as C. intermedius, until individuals occurring on Cerro Tancítaro in Michoacán, México, were reevaluated and classified as a new species (C. tancitarensis) based on scale pattern and geographic location. This study aimed to characterize the venom of C. tancitarensis and compare the venom profile to those of other species within the Crotalus intermedius group using gel electrophoresis, biochemical assays, reverse-phase high performance liquid chromatography, mass spectrometry, and lethal toxicity (LD50) assays. Results show that the venom profiles of species within the Crotalus intermedius group are similar, but with distinct differences in phospholipase A2 (PLA2), metalloproteinase PI (SVMP PI), and kallikrein-like serine proteinase (SVSP) activity and relative abundance. Proteomic analysis indicated that the highland forms produce venoms with 50-60 protein isoforms and a composition typical of type I rattlesnake venoms (abundant SVMPs, lack of presynaptic PLA2-based neurotoxins), as well as a diversity of typical Crotalus venom components such as serine proteinases, PLA2s, C-type lectins, and less abundant toxins (LAAOs, CRiSPs, etc.). The overall venom profile of C. tancitarensis appears most similar to C. transversus, which is consistent with a previous mitochondrial DNA analysis of the Crotalus intermedius group. These rattlesnakes of the Mexican highlands represent a radiation of high elevation specialists, and in spite of divergence of species in these Sky Island habitats, venom composition of species analyzed here has remained relatively conserved. The majority of protein family isoforms are conserved in all members of the clade, and as seen in other more broadly distributed rattlesnake species, differences in their venoms are largely due to relative concentrations of specific components.
Keywords: RP-HPLC; SDS-PAGE; enzymes; evolution; mass spectrometry; phenotype; snake venom metalloproteinase; toxin; venom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Review of Rattlesnake Venoms.Toxins (Basel). 2023 Dec 19;16(1):2. doi: 10.3390/toxins16010002. Toxins (Basel). 2023. PMID: 38276526 Free PMC article. Review.
-
Venom Ontogeny in the Mexican Lance-Headed Rattlesnake (Crotalus polystictus).Toxins (Basel). 2018 Jul 3;10(7):271. doi: 10.3390/toxins10070271. Toxins (Basel). 2018. PMID: 29970805 Free PMC article.
-
Proteomic, toxicological and immunogenic characterization of Mexican west-coast rattlesnake (Crotalus basiliscus) venom and its immunological relatedness with the venom of Central American rattlesnake (Crotalus simus).J Proteomics. 2017 Mar 31;158:62-72. doi: 10.1016/j.jprot.2017.02.015. Epub 2017 Feb 24. J Proteomics. 2017. PMID: 28238904
-
Comparison of venom composition and biological activities of the subspecies Crotalus lepidus lepidus, Crotalus lepidus klauberi and Crotalus lepidus morulus from Mexico.Toxicon. 2013 Sep;71:84-95. doi: 10.1016/j.toxicon.2013.05.006. Epub 2013 May 31. Toxicon. 2013. PMID: 23732126
-
Antitumoral activity of snake venom proteins: new trends in cancer therapy.Biomed Res Int. 2014;2014:203639. doi: 10.1155/2014/203639. Epub 2014 Feb 13. Biomed Res Int. 2014. PMID: 24683541 Free PMC article. Review.
Cited by
-
A Review of Rattlesnake Venoms.Toxins (Basel). 2023 Dec 19;16(1):2. doi: 10.3390/toxins16010002. Toxins (Basel). 2023. PMID: 38276526 Free PMC article. Review.
-
A Comparative Analysis of the Cytotoxic and Vascular Activity Effects of Western Diamondback Rattlesnake (Crotalus atrox) and Eastern Diamondback Rattlesnake (Crotalus adamanteus) Venoms Using a Chick Embryo Model.Animals (Basel). 2024 May 30;14(11):1634. doi: 10.3390/ani14111634. Animals (Basel). 2024. PMID: 38891681 Free PMC article.
References
-
- Uetz P., Freed P., Aguilar R., Hošek J., editors. The Reptile Database 2022. [(accessed on 30 August 2022)]. Available online: http://www.reptile-database.org.
-
- Bryson R.W., Murphy R.W., Graham M.R., Lathrop A., Lazcano D. Ephemeral Pleistocene woodlands connect the dots for highland rattlesnakes of the Crotalus intermedius group. J. Biogeog. 2011;38:2299–2310. doi: 10.1111/j.1365-2699.2011.02565.x. - DOI
-
- Colis-Torres A., Neri-Castro E., Strickland J.L., Olvera-Rodríguez A., Borja M., Calvete J., Jones J., Parkinson C.L., Bañuelos J., López de León J., et al. Intraspecific venom variation of Mexican West Coast Rattlesnakes (Crotalus basiliscus) and its implications for antivenom production. Biochimie. 2022;192:111–124. doi: 10.1016/j.biochi.2021.10.006. - DOI - PubMed
-
- Durban J., Sanz L., Trevisan-Silva D., Neri-Castro E., Alagón A., Calvete J.J. Integrated venomics and venom gland transcriptome analysis of juvenile and adult Mexican rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus revealed miRNA-modulated ontogenetic shifts. J. Proteome Res. 2017;16:3370–3390. doi: 10.1021/acs.jproteome.7b00414. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

