Development of In Vitro and Ex Vivo Biofilm Models for the Assessment of Antibacterial Fibrous Electrospun Wound Dressings
- PMID: 36669095
- PMCID: PMC9907351
- DOI: 10.1021/acs.molpharmaceut.2c00902
Development of In Vitro and Ex Vivo Biofilm Models for the Assessment of Antibacterial Fibrous Electrospun Wound Dressings
Abstract
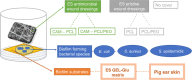
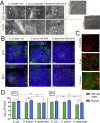
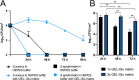
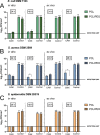
Increasing evidence suggests that the chronicity of wounds is associated with the presence of bacterial biofilms. Therefore, novel wound care products are being developed, which can inhibit biofilm formation and/or treat already formed biofilms. A lack of standardized assays for the analysis of such novel antibacterial drug delivery systems enhances the need for appropriate tools and models for their characterization. Herein, we demonstrate that optimized and biorelevant in vitro and ex vivo wound infection and biofilm models offer a convenient approach for the testing of novel antibacterial wound dressings for their antibacterial and antibiofilm properties, allowing one to obtain qualitative and quantitative results. The in vitro model was developed using an electrospun (ES) thermally crosslinked gelatin-glucose (GEL-Glu) matrix and an ex vivo wound infection model using pig ear skin. Wound pathogens were used for colonization and biofilm development on the GEL-Glu matrix or pig skin with superficial burn wounds. The in vitro model allowed us to obtain more reproducible results compared with the ex vivo model, whereas the ex vivo model had the advantage that several pathogens preferred to form a biofilm on pig skin compared with the GEL-Glu matrix. The in vitro model functioned poorly for Staphylococcus epidermidis biofilm formation, but it worked well for Escherichia coli and Staphylococcus aureus, which were able to use the GEL-Glu matrix as a nutrient source and not only as a surface for biofilm growth. On the other hand, all tested pathogens were equally able to produce a biofilm on the surface of pig skin. The developed biofilm models enabled us to compare different ES dressings [pristine and chloramphenicol-loaded polycaprolactone (PCL) and PCL-poly(ethylene oxide) (PEO) (PCL/PEO) dressings] and understand their biofilm inhibition and treatment properties on various pathogens. Furthermore, we show that biofilms were formed on the wound surface as well as on a wound dressing, indicating that the demonstrated methods mimic well the in vivo situation. Colony forming unit (CFU) counting and live biofilm matrix as well as bacterial DNA staining together with microscopic imaging were performed for biofilm quantification and visualization, respectively. The results showed that both wound biofilm models (in vitro and ex vivo) enabled the evaluation of the desired antibiofilm properties, thus facilitating the design and development of more effective wound care products and screening of various formulations and active substances.
Keywords: antibacterial; antibiofilm; electrospinning; ex vivo biofilm model; in vitro biofilm model; skin wound infection; wound dressings.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Stuermer E. K.; Besser M.; Brill F.; Geffken M.; Plattfaut I.; Severing A. L.; Wiencke V.; Rembe J. D.; Naumova E. A.; Kampe A.; Debus S.; Smeets R. Comparative Analysis of Biofilm Models to Determine the Efficacy of Antimicrobials. Int. J. Hyg. Environ. Health 2021, 234, 113744 10.1016/j.ijheh.2021.113744. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

