Randomized Phase III Trial of Ganitumab With Interval-Compressed Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma: A Report From the Children's Oncology Group
- PMID: 36669140
- PMCID: PMC10082251
- DOI: 10.1200/JCO.22.01815
Randomized Phase III Trial of Ganitumab With Interval-Compressed Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma: A Report From the Children's Oncology Group
Abstract
Purpose: Monoclonal antibodies directed against insulin-like growth factor-1 receptor (IGF-1R) have shown activity in patients with relapsed Ewing sarcoma. The primary objective of Children's Oncology Group trial AEWS1221 was to determine if the addition of the IGF-1R monoclonal antibody ganitumab to interval-compressed chemotherapy improves event-free survival (EFS) in patients with newly diagnosed metastatic Ewing sarcoma.
Methods: Patients were randomly assigned 1:1 at enrollment to standard arm (interval-compressed vincristine/doxorubicin/cyclophosphamide alternating once every 2 weeks with ifosfamide/etoposide = VDC/IE) or to experimental arm (VDC/IE with ganitumab at cycle starts and as monotherapy once every 3 weeks for 6 months after conventional therapy). A planned sample size of 300 patients was projected to provide 81% power to detect an EFS hazard ratio of 0.67 or smaller for the experimental arm compared with the standard arm with a one-sided α of .025.
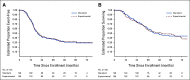
Results: Two hundred ninety-eight eligible patients enrolled (148 in standard arm; 150 in experimental arm). The 3-year EFS estimates were 37.4% (95% CI, 29.3 to 45.5) for the standard arm and 39.1% (95% CI, 31.3 to 46.7) for the experimental arm (stratified EFS-event hazard ratio for experimental arm 1.00; 95% CI, 0.76 to 1.33; 1-sided, P = .50). The 3-year overall survival estimates were 59.5% (95% CI, 50.8 to 67.3) for the standard arm and 56.7% (95% CI, 48.3 to 64.2) for the experimental arm. More cases of pneumonitis after radiation involving thoracic fields and nominally higher rates of febrile neutropenia and ALT elevation were reported on the experimental arm.
Conclusion: Ganitumab added to interval-compressed chemotherapy did not significantly reduce the risk of EFS event in patients with newly diagnosed metastatic Ewing sarcoma, with outcomes similar to prior trials without IGF-1R inhibition or interval compression. The addition of ganitumab may be associated with increased toxicity.
Trial registration: ClinicalTrials.gov NCT02306161.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


Comment in
-
Results from the Children's Oncology Group phase III trial of a monoclonal antibody against the insulin-like growth factor-1 receptor in patients with newly diagnosed metastatic Ewing sarcoma.Transl Pediatr. 2023 Oct 30;12(10):1916-1919. doi: 10.21037/tp-23-388. Epub 2023 Oct 10. Transl Pediatr. 2023. PMID: 37969125 Free PMC article. No abstract available.
References
-
- Grier HE, Krailo MD, Tarbell NJ, et al. : Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 348, 694-701, 2003 - PubMed
-
- Miser JS, Krailo MD, Tarbell NJ, et al. : Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: Evaluation of combination ifosfamide and etoposide—A Children's Cancer Group and Pediatric Oncology Group study. J Clin Oncol 22, 2873-2876, 2004 - PubMed
-
- Miser JS, Goldsby RE, Chen Z, et al. : Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: Evaluation of increasing the dose intensity of chemotherapy—A report from the Children's Oncology Group. Pediatr Blood Cancer 49, 894-900, 2007 - PubMed
-
- Ladenstein R, Potschger U, Le Deley MC, et al. : Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J Clin Oncol 28, 3284-3291, 2010 - PubMed
-
- Bernstein ML, Devidas M, Lafreniere D, et al. : Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children's Cancer Group phase II study 9457—A report from the Children's Oncology Group. J Clin Oncol 24, 152-159, 2006 - PubMed

