Dexrazoxane and Long-Term Heart Function in Survivors of Childhood Cancer
- PMID: 36669148
- PMCID: PMC10448941
- DOI: 10.1200/JCO.22.02423
Dexrazoxane and Long-Term Heart Function in Survivors of Childhood Cancer
Abstract
Purpose: For survivors of childhood cancer treated with doxorubicin, dexrazoxane is cardioprotective for at least 5 years. However, longer-term data are lacking.
Methods: Within the Children's Oncology Group and the Dana Farber Cancer Institute's Childhood Acute Lymphoblastic Leukemia Consortium, we evaluated four randomized trials of children with acute lymphoblastic leukemia or Hodgkin lymphoma, who received doxorubicin with or without dexrazoxane, and a nonrandomized trial of patients with osteosarcoma who all received doxorubicin with dexrazoxane. Cumulative doxorubicin doses ranged from 100 to 600 mg/m2 across these five trials, and dexrazoxane was administered uniformly (10:1 mg/m2 ratio) as an intravenous bolus before doxorubicin. Cardiac function was prospectively assessed in survivors from these trials, plus a matched group of survivors of osteosarcoma treated with doxorubicin without dexrazoxane. Two-dimensional echocardiograms and blood biomarkers were analyzed centrally in blinded fashion. Multivariate analyses adjusted for demographic characteristics, cumulative doxorubicin dose, and chest radiotherapy determined the differences and associations by dexrazoxane status.
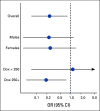
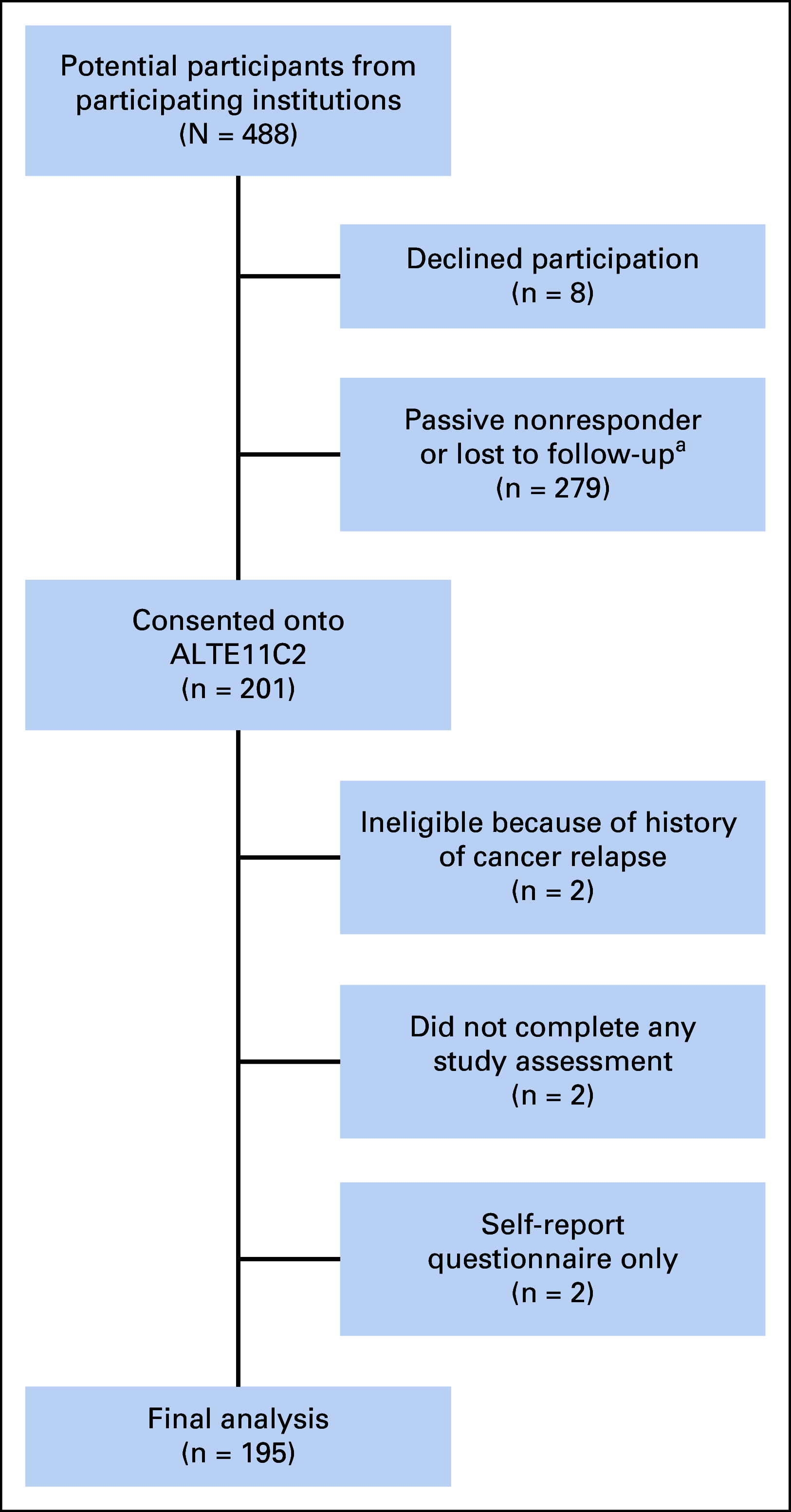
Results: From 49 participating institutions, 195 participants were assessed at 18.1 ± 2.7 years since cancer diagnosis (51% dexrazoxane-exposed; cumulative doxorubicin dose 297 ± 91 mg/m2). Dexrazoxane administration was associated with superior left ventricular fractional shortening (absolute difference, +1.4% [95% CI, 0.3 to 2.5]) and ejection fraction (absolute difference, +1.6% [95% CI, 0.0 to 3.2]), and lower myocardial stress per B-type natriuretic peptide (-6.7 pg/mL [95% CI, -10.6 to -2.8]). Dexrazoxane was associated with a reduced risk of having lower left ventricular function (fractional shortening < 30% or ejection fraction < 50%; odds ratio, 0.24 [95% CI, 0.07 to 0.81]). This protective association was primarily seen in those treated with cumulative doxorubicin doses ≥ 250 mg/m2.
Conclusion: Among young adult-aged survivors of childhood cancer, dexrazoxane was associated with a cardioprotective effect nearly 20 years after initial anthracycline exposure.
Trial registration: ClinicalTrials.gov NCT01790152.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
Similar articles
-
Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial.Lancet Oncol. 2010 Oct;11(10):950-61. doi: 10.1016/S1470-2045(10)70204-7. Epub 2010 Sep 16. Lancet Oncol. 2010. PMID: 20850381 Free PMC article. Clinical Trial.
-
Late health outcomes after dexrazoxane treatment: A report from the Children's Oncology Group.Cancer. 2022 Feb 15;128(4):788-796. doi: 10.1002/cncr.33974. Epub 2021 Oct 13. Cancer. 2022. PMID: 34644414 Free PMC article. Clinical Trial.
-
Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404.J Clin Oncol. 2016 Mar 10;34(8):854-62. doi: 10.1200/JCO.2015.60.8851. Epub 2015 Dec 23. J Clin Oncol. 2016. PMID: 26700126 Free PMC article. Clinical Trial.
-
Dexrazoxane. A review of its use as a cardioprotective agent in patients receiving anthracycline-based chemotherapy.Drugs. 1998 Sep;56(3):385-403. doi: 10.2165/00003495-199856030-00009. Drugs. 1998. PMID: 9777314 Review.
-
Exposure to anthracyclines during childhood causes cardiac injury.Semin Oncol. 2006 Jun;33(3 Suppl 8):S8-14. doi: 10.1053/j.seminoncol.2006.04.019. Semin Oncol. 2006. PMID: 16781284 Review.
Cited by
-
Late Cardiac Toxic Effects Associated With Treatment Protocols for Hodgkin Lymphoma in Children.JAMA Netw Open. 2024 Jan 2;7(1):e2351062. doi: 10.1001/jamanetworkopen.2023.51062. JAMA Netw Open. 2024. PMID: 38241048 Free PMC article. Clinical Trial.
-
Apelinergic System Affects Electrocardiographic Abnormalities Induced by Doxorubicin.Biomedicines. 2025 Jan 3;13(1):94. doi: 10.3390/biomedicines13010094. Biomedicines. 2025. PMID: 39857678 Free PMC article.
-
The cardio-oncologic burden of breast cancer: molecular mechanisms and importance of preclinical models.Basic Res Cardiol. 2025 Feb;120(1):91-112. doi: 10.1007/s00395-024-01090-w. Epub 2024 Dec 2. Basic Res Cardiol. 2025. PMID: 39621070 Free PMC article. Review.
-
Children's Oncology Group's 2023 blueprint for research: Hodgkin lymphoma.Pediatr Blood Cancer. 2023 Sep;70 Suppl 6(Suppl 6):e30580. doi: 10.1002/pbc.30580. Epub 2023 Jul 28. Pediatr Blood Cancer. 2023. PMID: 37505794 Free PMC article.
-
Doxorubicin-Induced Cardiotoxicity and the Emerging Role of SGLT2 Inhibitors: From Glycemic Control to Cardio-Oncology.Pharmaceuticals (Basel). 2025 May 3;18(5):681. doi: 10.3390/ph18050681. Pharmaceuticals (Basel). 2025. PMID: 40430500 Free PMC article. Review.
References
-
- Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2018. Bethesda, MD: National Cancer Institute; 2021.
-
- Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

