Lymphatic vessels in bone support regeneration after injury
- PMID: 36669473
- PMCID: PMC11913777
- DOI: 10.1016/j.cell.2022.12.031
Lymphatic vessels in bone support regeneration after injury
Abstract
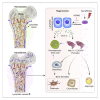
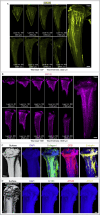
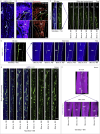
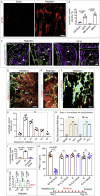
Blood and lymphatic vessels form a versatile transport network and provide inductive signals to regulate tissue-specific functions. Blood vessels in bone regulate osteogenesis and hematopoiesis, but current dogma suggests that bone lacks lymphatic vessels. Here, by combining high-resolution light-sheet imaging and cell-specific mouse genetics, we demonstrate presence of lymphatic vessels in mouse and human bones. We find that lymphatic vessels in bone expand during genotoxic stress. VEGF-C/VEGFR-3 signaling and genotoxic stress-induced IL6 drive lymphangiogenesis in bones. During lymphangiogenesis, secretion of CXCL12 from proliferating lymphatic endothelial cells is critical for hematopoietic and bone regeneration. Moreover, lymphangiocrine CXCL12 triggers expansion of mature Myh11+ CXCR4+ pericytes, which differentiate into bone cells and contribute to bone and hematopoietic regeneration. In aged animals, such expansion of lymphatic vessels and Myh11-positive cells in response to genotoxic stress is impaired. These data suggest lymphangiogenesis as a therapeutic avenue to stimulate hematopoietic and bone regeneration.
Keywords: 3D imaging; IL6; aging; bone; hematopoiesis; injury; lymphangiocrine; lymphatic vessels; regeneration; stress.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors do not declare competing financial interests.
Figures










Comment in
-
Lymphatic vessels identified in bones.Nat Cardiovasc Res. 2023 Mar;2(3):223. doi: 10.1038/s44161-023-00255-5. Nat Cardiovasc Res. 2023. PMID: 39196023 No abstract available.
References
-
- Guo P., Poulos M.G., Palikuqi B., Badwe C.R., Lis R., Kunar B., Ding B.S., Rabbany S.Y., Shido K., Butler J.M., Rafii S. Endothelial jagged-2 sustains hematopoietic stem and progenitor reconstitution after myelosuppression. J. Clin. Invest. 2017;127:4242–4256. doi: 10.1172/jci92309. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

