Long-term Pegylated GH for Children With GH Deficiency: A Large, Prospective, Real-world Study
- PMID: 36669772
- PMCID: PMC10348466
- DOI: 10.1210/clinem/dgad039
Long-term Pegylated GH for Children With GH Deficiency: A Large, Prospective, Real-world Study
Abstract
Context: The evidence of long-term polyethylene glycol recombinant human GH (PEG-rhGH) in pediatric GH deficiency (GHD) is limited.
Objective: This study aimed to examine the effectiveness and safety of long-term PEG-rhGH in children with GHD in the real world, as well as to examine the effects of dose on patient outcomes.
Design: A prospective, observational, posttrial study (NCT03290235).
Setting, participants and intervention: Children with GHD were enrolled from 81 centers in China in 4 individual clinical trials and received weekly 0.2 mg/kg/wk (high-dose) or 0.1 to <0.2 mg/kg/wk (low-dose) PEG-rhGH for 30 months.
Main outcomes measures: Height SD score (Ht SDS) at 12, 24, and 36 months.
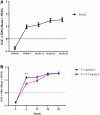
Results: A total of 1170 children were enrolled in this posttrial study, with 642 patients in the high-dose subgroup and 528 in the low-dose subgroup. The Ht SDS improved significantly after treatment in the total population (P < 0.0001), with a mean change of 0.53 ± 0.30, 0.89 ± 0.48, 1.35 ± 0.63, 1.63 ± 0.75 at 6 months, 12 months, 24 months, and 36 months, respectively. In addition, the changes in Ht SDS from baseline were significantly improved in the high-dose subgroup compared with the low-dose subgroup at 6, 12, 24, and 36 months after treatment (all P < 0.05). A total of 12 (1.03%) patients developed serious adverse events. There was no serious adverse event related to the treatment, and no AEs leading to treatment discontinuation or death occurred.
Conclusions: PEG-rhGH showed long-term effectiveness and safety in treating children with GHD. Both dose subgroups showed promising outcomes, whereas PEG-rhGH 0.2 mg/kg/wk might show additional benefit.
Keywords: Jintrolong; PEGylated recombinant human growth hormone; growth hormone deficiency; long-acting growth hormone.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Conflict of interest statement
The corresponding author obtained the relevant information from all co-authors. The authors declare that there is no conflict of interest.
Figures



References
-
- Hage C, Gan HW, Ibba A, et al. . Advances in differential diagnosis and management of growth hormone deficiency in children. Nat Rev Endocrinol. 2021;17(10):608‐624. - PubMed
-
- Bao XL, Shi YF, Du YC, Liu R, Deng JY, Gao SM. Prevalence of growth hormone deficiency of children in Beijing. Chin Med J (Engl). 1992; 105(5):401‐405. - PubMed
-
- Wang C, Huang H, Zhao C, et al. . The impact of pegylated recombinant human growth hormone replacement therapy on glucose and lipid metabolism in children with growth hormone deficiency. Ann Palliat Med. 2021;10(2):1809‐1814. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 2018YFC1002400/The National Key Research and Development Program of China is funded by Ministry of Science and Technology of the People's Republic of China
- ZDZX2020000020/The Special Science and Technology Major Project of Hubei Province is funded by Hubei Provincial Science and Technology Department
LinkOut - more resources
Full Text Sources
Medical

